What is an atrial septal defect (ASD)?
What are the known causes of ASD?
What symptoms are characteristic of an atrial septal defect?
How can an ASD be diagnosed?
What treatment methods for ASD in children and adults are currently used?
What types of surgeries are used to treat ASD?
What drugs can be used in the treatment of atrial septal defect?
How should nutritional deficiencies be compensated for in children with ASD?
How does the postoperative period proceed after surgery to close an atrial septal defect?
What is the prognosis after surgery for atrial septal defect?
Is it possible to prevent the development of ASD and what should parents do when a child is diagnosed?
What is an atrial septal defect (ASD)?
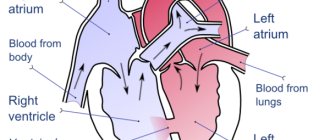
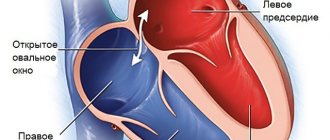
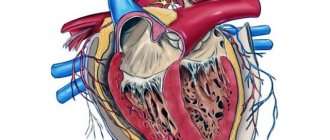
An atrial septal defect is an abnormal hole in the muscle wall located between the two atria (upper chambers) of the heart. During embryonic development, to ensure proper blood circulation in the heart, an opening is formed between the right and left atria. It is also called the open foramen ovale and is necessary to balance the blood flow in the child’s systemic and pulmonary circulation, since due to the lack of breathing and gas exchange, the small circle is not sufficiently active. Usually it heals on its own immediately before birth. But in some cases, an open foramen ovale or, if it is larger, an atrial septal defect persists and causes a pathological discharge of blood from the left atrium to the right or so-called blood shunting occurs.
Depending on the location of the defect in the interatrial septum, several variants of ASD are distinguished. Most often, the pathological communication between the atria is located in the center or middle of the interatrial septum, which, according to statistics, accounts for more than 70% of all cases of ASD. According to the classification of ASD, there are 4 more forms of defects:
- a pathological hole is formed in the upper part of the septum in the area where the superior vena cava enters the right atrium, which is also called an atrial septal defect of the venous sinus;
- in the lower part of the septum, in the area of the confluence of the inferior vena cava;
- in the lowest part of the septum between the atria directly above the ventricles and is called a primary ASD;
- multiple atrial septal defects.
Depending on the location of these pathological messages, they may influence the intracardiac circulation. For example, an ASD of the sinus venosus can involve the right superior pulmonary vein, and a primary ASD is often detected in combination with a disorder of the mitral valve and/or tricuspid valve of the heart and causes regurgitation (backflow of blood) through these valves. In some cases, it is possible to form a combined congenital heart defect, for example, a combination with a ventricular septal defect. ASDs in children can be either small, point-sized or large, involving almost the entire septum.
Atrial and ventricular septal defects are the most common congenital heart defects. ASDs in children account for approximately 4-10% of all congenital heart defects in the United States. Atrial septal defect is 2 times more common in women.
Fig. 1 The most common types of ASD in children (explanation in the text)
Congenital anomalies [malformations] of the cardiac septum (Q21)
Excludes: acquired cardiac septal defect (I51.0)
Coronary sinus defect
Unclosed or preserved:
- foramen ovale
- secondary hole (type II)
Venous sinus defect
Common atrioventricular canal
Endocardial defect at the base of the heart
Atrial septal defect (type II)
Ventricular septal defect with pulmonary stenosis or artesia, aortic dextroposition and right ventricular hypertrophy.
Aortic septal defect
Eisenmenger's defect
Septal defect (heart) NOS
In Russia, the International Classification of Diseases, 10th revision (ICD-10) has been adopted as a single normative document for recording morbidity, reasons for the population's visits to medical institutions of all departments, and causes of death.
ICD-10 was introduced into healthcare practice throughout the Russian Federation in 1999 by order of the Russian Ministry of Health dated May 27, 1997. No. 170
The release of a new revision (ICD-11) is planned by WHO in 2017-2018.
https://www.youtube.com/watch?v=kEGKCuI0ni4
With changes and additions from WHO.
RCHR (Republican Center for Health Development of the Ministry of Health of the Republic of Kazakhstan)
Version: Clinical protocols of the Ministry of Health of the Republic of Kazakhstan
Congenital heart defects are one of the most common developmental anomalies, ranking third after anomalies of the central nervous system and musculoskeletal system. The birth rate of children with congenital heart defects in all countries of the world ranges from 2.4 to 14.2 per 1000 newborns. The incidence of congenital heart defects among live births is 0.
7-1.2 per 1000 newborns. Defects with the same frequency of occurrence are often presented differently in the nosological structure among patients admitted to cardiology departments (for example, a small atrial septal defect and tetralogy of Fallot). This is due to varying degrees of threat to the health or life of the child.
Problems of diagnosis and treatment of congenital heart defects are extremely important in pediatric cardiology. Therapists and cardiologists, as a rule, are not sufficiently familiar with this pathology due to the fact that the vast majority of children by the age of puberty undergo surgical treatment or die without receiving timely and adequate help.
The causes of congenital heart defects are unclear. The most vulnerable period is during pregnancy, that is, the time when the formation and formation of heart structures occurs. Teratogenic environmental factors, diseases of the mother and father, infections, especially viral ones, as well as parental alcoholism, drug use, and maternal smoking are of great importance. Many chromosomal diseases are associated with congenital heart defects.
Anatomical and morphological severity, i.e. type of pathology. The following prognostic groups are distinguished:
- congenital heart defects with a relatively favorable outcome: patent ductus arteriosus, ventricular septal defect (VSD), atrial septal defect (ASD), pulmonary stenosis; with these defects, the natural mortality rate in the first year of life is 8-11%;
- tetralogy of Fallot, natural mortality in the first year of life%;
- complex congenital heart defects: left ventricular hypoplasia, pulmonary atresia, common truncus arteriosus; natural mortality in the first year of life - from 36-52% to 73-97%.
- The age of the patient at the time of manifestation of the defect (appearance of clinical signs of hemodynamic disorders).
- The presence of other (extracardiac) malformations increases mortality in a third of children with congenital heart disease to 90%.
- Birth weight and prematurity.
- Age of the child at the time of correction of the defect.
- The severity and degree of hemodynamic changes, in particular the degree of pulmonary hypertension.
- Type and option of cardiac surgery.
Without surgical treatment, congenital heart defects develop differently. For example, in children aged 2-3 weeks, hypoplastic left heart syndrome or pulmonary atresia (with an intact atrial septum) are rarely observed, which is associated with high early mortality in such defects. The overall mortality rate for congenital heart defects is high.
By the end of the first week, 29% of newborns die, by the first month - 42%, by the first year - 87% of children. Taking into account the modern possibilities of cardiac surgery, for almost all congenital heart defects it is possible to perform surgery on a newborn. However, not all children with congenital heart defects require surgical treatment immediately after the pathology is detected.
Taking into account treatment tactics, patients with congenital heart defects are divided into three groups:
- patients for whom surgery for congenital heart defects is necessary and possible (about 52%);
- patients for whom surgery is not indicated due to minor hemodynamic disturbances (about 31%);
- patients for whom correction of congenital heart defects is impossible, as well as inoperable due to their somatic condition (about 17%).
A doctor who suspects a congenital heart defect faces the following tasks:
- identification of symptoms indicating the presence of congenital heart disease;
- carrying out differential diagnosis with other diseases that have similar clinical manifestations;
- resolving the issue of the need for urgent consultation with a specialist (cardiologist, cardiac surgeon);
- conducting pathogenetic therapy.
There are more than 90 types of congenital heart defects and many combinations of them.
When interviewing parents, you should clarify the timing of the development of the child’s static functions: when he began to sit independently in a crib and walk. It is necessary to find out how the child gained weight in the first year of life, since heart failure and hypoxia accompanying heart defects are accompanied by increased fatigue, “lazy” sucking and poor weight gain.
Defects with hypervolemia of the pulmonary circulation may be accompanied by frequent pneumonia and bronchitis. If you suspect a defect with cyanosis, you should clarify the timing of the appearance of cyanosis (from birth or during the first six months of life), under what circumstances cyanosis appears, and its localization. In addition, with defects with cyanosis, there is always polycythemia, which can be accompanied by disorders of the central nervous system, such as hyperthermia, hemiparesis, and paralysis.
Body type
Changes in physique occur with few defects. Thus, coarctation of the aorta is accompanied by the formation of an “athletic” physique, with a predominance of the development of the shoulder girdle. In most cases, congenital heart defects are characterized by low nutrition, often up to the 1st degree of malnutrition, and/or hypostatura.
It is possible to develop symptoms such as “drumsticks” and “watch glasses”, which is typical for congenital heart defects of the blue type.
Skin
For pale type defects - paleness of the skin; for defects with cyanosis - diffuse cyanosis of the skin and visible mucous membranes, with a predominance of acrocyanosis. However, the rich “crimson” color of the terminal phalanges is also characteristic of high pulmonary hypertension, which accompanies defects with left-to-right blood discharge.
Respiratory system
Changes in the respiratory system often reflect a state of increased pulmonary blood flow and manifest themselves in the early stages as shortness of breath and signs of dyspnea.
On examination, a “heart hump” is determined, located bisternally or on the left. On palpation - the presence of systolic or diastolic tremors, pathological cardiac impulse. Percussion - changes in the boundaries of relative dullness of the heart. During auscultation - in what phase of the cardiac cycle the murmur is heard, its duration (what part of systole, diastole occupies), the variability of the murmur when changing body position, the conductivity of the murmur.
Changes in blood pressure (BP) with congenital heart disease are uncommon. Thus, coarctation of the aorta is characterized by an increase in blood pressure in the arms and a significant decrease in the legs. However, similar changes in blood pressure can also occur with vascular pathology, in particular with nonspecific aortoarteritis. In the latter case, significant asymmetry of blood pressure is possible on the right and left arm, on the right and left leg. A decrease in blood pressure can occur with defects with severe hypovolemia, for example, with aortic stenosis.
With congenital heart disease, enlargement of the liver and spleen is possible due to venous stagnation in heart failure (usually no more than 1.5-2 cm). Venous congestion of the vessels of the mesentery and esophagus may be accompanied by vomiting, more often during physical exertion, and abdominal pain (due to stretching of the liver capsule).
There are several classifications of congenital heart defects.
International Classification of Diseases, 10th revision. Congenital heart defects fall under Q20-Q28. Classification of heart disease in children (WHO, 1970) with SNOP (Systematic Nomenclature of Pathology) codes used in the USA and International Society of Cardiology (ISC) codes.
Classification of congenital defects of the heart and blood vessels (WHO, 1976), containing the section “Congenital anomalies (developmental defects)” with the headings “Anomalies of the bulb of the heart and anomalies of the closure of the cardiac septum”, “Other congenital anomalies of the heart”, “Other congenital anomalies of the circulatory system”.
Creating a unified classification presents certain difficulties due to the large number of types of congenital heart defects, as well as the difference in principles that can be used as the basis for the classification. At the Scientific Center for Cardiovascular Surgery named after. A.N. Bakulev developed a classification in which congenital heart defects are divided into groups taking into account anatomical features and hemodynamic disorders. The proposed classification is convenient for use in practice. In this classification, all congenital heart defects are divided into three groups:
- Congenital heart disease of the pale type with an arteriovenous shunt, i.e. with blood discharge from left to right (VSD, ASD, patent ductus arteriosus);
- Blue-type congenital heart disease with venoarterial discharge, i.e. with blood discharge from right to left (complete transposition of the great vessels, tetralogy of Fallot);
- CHD without discharge, but with an obstruction to ejection from the ventricles (pulmonary artery stenosis, coarctation of the aorta).
What are the known causes of ASD?
The cause of the formation of an atrial septal defect is the peculiarities of the embryonic development of the heart. It is difficult to clearly identify the reasons why this occurs and which factors can influence this process, but in most cases, experts agree that genetic, environmental, physical and infectious factors are to blame for the appearance of ASD. In cases where the ASD turns out to be small and there are no symptoms, it may go completely unnoticed by both the patient and doctors. If the defect turns out to be large, oxygenated blood from the left atrium enters the right atrium and then back to the lungs. The heart has to pump extra blood and do “unnecessary” work. As a result of this stress, the right side of the heart can increase in size and heart failure may occur.
In some cases, a patent foramen ovale or atrial septal defect can cause blood clots to move from the right side of the heart to the left, which can result in migration of this blood clot from the heart to the brain, followed by the development of a stroke . If left untreated, ASD may develop into a complicated course with symptoms of pulmonary hypertension , lung infection, Eisenmenger syndrome, atrial fibrillation , atrial flutter , stroke, or right-sided heart failure.
Small anomalies of heart development
Small anomalies of heart development: Brief description
Minor anomalies of cardiac development (MADC) are anatomical congenital changes in the heart and great vessels that do not lead to gross dysfunction of the cardiovascular system. A number of MARS are unstable and disappear with age.
Etiology
Hereditary determined connective tissue dysplasia. A number of MARS are dysembryogenetic in nature. The influence of various environmental factors (chemical, physical effects) cannot be excluded.
Code according to the international classification of diseases ICD-10:
- Q20. 9 - Congenital anomaly of cardiac chambers and connections, unspecified
What symptoms are characteristic of an atrial septal defect?
Due to good compensatory capabilities or a small diameter of the atrial septal defect, symptoms of ASD may be completely absent until entering adulthood. One of the first symptoms of ASD in this case is a slowdown in the child’s physical development compared to peers. Among other symptoms that also do not appear immediately are the following:
- dyspnea
- general unmotivated weakness
- abnormal heart rhythm (arthymia) or episodes of heart fluttering,
- fatigue during physical activity, the need to stop during training or exercise
- difficulty breathing during exercise or physical activity
It should be noted that the older the patient gets, the more intense the severity of these ASD symptoms becomes (of course, in the absence of other diseases).
For what symptoms of ASD should you call a doctor?
There are a number of conditions in which the child’s parents need to call a pediatrician or pediatric cardiologist to their home:
- swelling or swelling in the ankles and lower legs
- increase in abdominal size
- exercise and exercise intolerance
- frequent and chronic bronchitis and respiratory tract infections
- change in normal blood pressure
- the addition of symptoms of a systemic infection in the body, including sore throat, body aches or fever
Sometimes the child's condition may worsen and specialized care will be required. Here are some of the conditions in which the child’s parents will need to call an ambulance or the “03” team to hospitalize the child in a specialized department:
- severe difficulty breathing or shortness of breath, the appearance of rapid shallow breathing
- dizziness or severe weakness
- persistent cough or cough that is bloody or streaked with blood
- bluish skin tone or bluish discoloration around the lips, nails, and tongue
- irregular heart rhythm (arrhythmia) or heart flutter,
- chest pain (rare in children)
Clinical picture of a dangerous condition
At the very beginning of its development, the anomaly is not accompanied by any signs and does not manifest itself. Further, its symptoms are most often associated with age:
- from 1 year to 3 years: attention should be paid to the appearance of some delay in the physical development of the baby; he may not have time to gain the required weight, or be too susceptible to viral infections;
- from 4 to 7 years: the child cannot withstand physical activity, complains of weakness, chest pain, and is stunted. Pallor of the skin and arrhythmias are observed;
- after 7 years: children of this age are also lagging behind in physical development; there may be a delay in the development of the reproductive system, chest pain. When listening, the doctor hears characteristic abnormalities: soft systolic murmurs.
- sudden chest pain;
- feeling of discomfort;
- increased weakness;
- inability to cope with any physical activity.
How can an ASD be diagnosed?
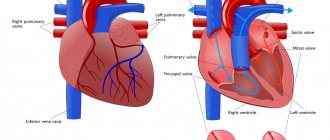
More information about the possible presence of congenital heart disease in general and atrial septal defect in particular can be obtained by carefully collecting a family history of congenital pathology in close relatives. A history of congenital heart disease in parents, brothers or sisters suggests the existence of a genetic predisposition . Already at an appointment with a pediatric cardiologist, a preliminary diagnosis can be established. To do this, the cardiologist will examine the child, measure blood pressure and auscultate (listen to) the heart and lungs using a stethoscope. Auscultation will allow you to listen to pathological murmurs in the heart caused by improper blood flow that occurs in the presence of an atrial septal defect. During the examination, the cardiologist will also evaluate the characteristics of the pulse, reflexes, height and weight of the baby, which will identify possible disturbances in heart rhythm and physical development. Because the circulatory problems that occur with ASD are often characterized by changes in the amount of oxygen in the blood, your child will have their oxygen saturation measured by placing a device called a pulse oximeter on the tip of their finger or earlobe. Also, during the examination, the cardiologist can palpate (feel) the internal organs in order to determine whether they are enlarged or painful.
A comprehensive diagnosis of ASD also includes such research methods as chest X-ray, electrocardiography (ECG, cardiogram), echocardiography (EchoCG) or magnetic resonance imaging (MRI), which can confirm the presence of a defect in the interatrial septum. A chest x-ray is used to evaluate the size, shape, and location of the heart and lungs.
An ECG displays the electrical activity of the heart. During an ECG examination, special small electrodes in the form of suction cups are attached to the surface of the skin on the front surface of the chest. These electrodes are connected by wires to an electrocardiograph, which recognizes and records in the form of a curve on paper the electrical impulses that arise in the heart at different stages of its contractile activity.
The principle of operation of EchoCG is to use the diagnostic properties of ultrasound or high-frequency sound waves, with the help of which computer analysis and construction of images of the internal structures of the heart are possible. With the help of echocardiography, any pathology of heart structures (valves, chambers, large vessels, etc.) can be determined with a high degree of reliability and accuracy. If echocardiography is performed in combination with Doppler sonography, it is possible to study the nature of blood flows occurring in the cavities of the heart and as it passes through the valve structures.
Echocardiography for atrial septal defect (video)
Magnetic resonance imaging is a research method that involves using the energy of a magnetic field and radio waves generated under its influence, which allows you to recreate a three-dimensional (3D) picture of the structure of the heart, and the use of a dynamic mode during an MRI study allows you to understand how efficiently the heart works and how circulation occurs blood inside the heart and large vessels.
If it is necessary to carry out additional diagnostics or determine treatment tactics for ASD, the child is hospitalized in a cardiac surgery center and, already in a hospital setting, undergoes a procedure called cardiac catheterization . This research method is more aggressive than those previously announced, since it involves performing a puncture of blood vessels and introducing special endovascular (intravascular) devices into the lumen of the vessel and the cavities of the heart. Cardiac catheterization is the “gold standard” for diagnosing ASD when planning surgical treatment. It is usually performed in a large, technically well-equipped cardiac surgery hospital, which has extensive experience in conducting such procedures and highly qualified staff. During cardiac catheterization, a long plastic elastic tube called a catheter is inserted into the lumen of a vein or artery by puncture and moved towards the heart under the control of an x-ray unit. After reaching the area of intended study (for example, the area of the interatrial communication with ASD), to improve the quality of the image and stain the vascular bed and cavities of the heart, a radiopaque material (contrast) is introduced into the lumen, followed by recording the image using an X-ray machine and recording the result on video. This research technique is also called angiography. The catheter also allows you to measure the pressure inside the cavities of the heart, as well as determine the saturation of blood obtained from different chambers of the heart. For example, if there is a communication between the atria, then oxygen-rich blood from the left atrium enters the right atrium, mixing there with oxygen-depleted venous blood. An increase in blood saturation in the right parts (atrium and ventricle) of the heart will indirectly indicate the presence of such a pathological communication between the right and left chambers of the heart.
Diagnostics
The following research methods allow you to check the condition and functioning of the heart and identify the disease:
- Electrocardiogram (ECG). Allows you to determine congestion of the cardiac ventricles, identify the presence and degree of pulmonary hypertension;
- Phonocardiography (PCG). As a result of the study, it is possible to detect heart murmurs;
- Echocardiography (EchoCG). Able to detect blood flow disturbances and help to suspect VSD;
- Ultrasonography. Helps evaluate the work of the myocardium, the pressure level of the pulmonary artery, the amount of blood discharged;
- Radiography. Chest photographs can be used to determine changes in the pulmonary pattern and an increase in the size of the heart;
- Probing of the heart. Allows you to determine the level of pressure in the arteries of the lungs and the ventricle of the heart, the increased oxygen content in venous blood;
- Pulse oximetry. Helps determine the level of oxygen in the blood - a deficiency indicates disorders in the cardiovascular system;
- Cardiac catheterization. Helps assess the condition of the heart structure and determine the level of pressure in the heart ventricles.
What treatment methods for ASD in children and adults are currently used?
As statistical studies have shown, in approximately 20% of children with an atrial septal defect, by the age of 2 years the defect closes on its own without treatment. In cases where spontaneous closure of the ASD does not occur, surgical treatment is usually undertaken, since further preservation of the defect is fraught with the formation of thickening of the walls of the pulmonary artery and pulmonary hypertension. If ASD is not treated promptly, an irreversible condition, also called chronic obstructive pulmonary artery disease, may develop, and the risk of an unfavorable outcome increases by 25%. The first stage of treatment for ASD in children is usually performed by a pediatric cardiologist who has extensive experience in diagnosing and treating congenital heart defects. It involves maintenance therapy and allows you to cope with circulatory problems and heart failure that have arisen, as well as prepare the child for surgery. Surgery for this defect is usually performed by an experienced pediatric cardiovascular surgeon with extensive experience in ASD closure and repair. Various types of endovascular interventions in the treatment of atrial septal defect are performed by an interventional cardiologist or a pediatric endovascular cardiac surgeon.
Fig. 2 Options for surgery for ASD (above - closing the defect with an occluder; below - repair with a patch)
What types of surgeries are used to treat ASD?
Open ASD surgery
There are 2 main options for closing an ASD during surgery: the first is used for a small size of the ASD and involves the application of one suture to bring the edges of this defect closer and aligned; during the second option, ASD plastic surgery is performed using a patch and its essence consists of replacing the defect area with a synthetic material (usually a Dacron patch) or with your own tissues (more often, a patch from the pericardium or a specially treated pericardium, called xenopericardium, is used for this).
During traditional surgery to close an ASD, access to the heart is performed either in the midline of the sternum with its subsequent dilation (the access is called sternotomy), or in one of the intercostal spaces (the access is called thoracotomy). After special preparation, the heart is connected to a heart-lung machine (heart-lung machine), the heart is cooled and stopped, and then ASD repair is performed under the conditions of a stationary and “dry” bloodless heart, which is necessary to perform high-precision surgical work. The recovery period after this treatment option for atrial septal defect is usually 5-7 days, and the rehabilitation period at home after discharge from the hospital is 4-6 weeks. Currently, to reduce recovery and rehabilitation time and reduce the size of scars, minimally invasive surgical techniques are used using small accesses up to 7-10 cm in length. The approach is usually chosen depending on the size of the ASD and its location. Surgery for asymptomatic cases of the disease is usually recommended 1-2 years before school. If there are symptoms and the child’s physical development is slow, it is recommended to treat the atrial septal defect as early as possible, before secondary changes appear in the heart and lungs.
Endovascular closure of ASD with an occluder
Endovascular ASD closure surgery has several advantages over traditional open surgery, making it increasingly popular and used as an alternative. First of all, endovascular treatment of atrial septal defect in children does not require wide traumatic access and the use of a heart-lung machine during the operation. As a result, this leads to a reduction in the length of stay in the hospital, the absence of large scars after surgery, and shortens the recovery and rehabilitation time. After endovascular closure of the ASD, the child remains in the hospital for one, maximum two days, and within 1-2 weeks there is a complete recovery and return to normal activity.
The endovascular treatment procedure is based on a kind of blockage of the pathological communication between the atria with a special device called an occluder. However, installation of an occluder for ASD is not always feasible. There are a number of clinical situations in which this treatment option for atrial septal defect is not entirely suitable, namely in cases where the communication between the atria is too large or not located in the center of the interatrial septum, or there is insufficient septal tissue to firmly fix the occluder. There are also a number of contraindications to closing an ASD using an occluder: severe vasoconstriction and inability to deliver the occluder to the implantation site; pathology of the heart valves, when correction of this pathology is necessary; pathological venous drainage from the lungs; presence of valve infection; the presence of blood clots in the lumen of the heart chambers or disorders of hemostasis (blood coagulation and anticoagulation system) or intolerance to aspirin or aspirin-like drugs.
The principle of endovascular closure of ASD in children (video)
That is why, before planning treatment for an atrial septal defect, an accurate diagnosis is carried out to determine the exact size and location of the defect. On the eve of the procedure for closing the ASD with an occluder, a course of anticoagulant and antiplatelet therapy is prescribed, which increases blood flow and reduces the possibility of thrombus formation. Before installation, the occluder is placed by the manufacturer in a special catheter, which, after puncture of the femoral vein, is moved to the location of the ASD. After which the device is implanted into the defect and it remains there permanently, preventing the mixing of different blood flows between the two atria. In order to determine the correct location and position of the occluder, control angiography is performed. Within 24 hours, control ECG and EchoCG studies are performed, as well as a chest x-ray, which also confirms the correct location of the occluder. Subsequently, in the long-term period after the ASD closure operation, the myocardial tissue grows into the device and it becomes part of the heart septum. Despite the fact that the occluder has clear dimensions and does not increase over time, as the child grows, the tissue covering the device also grows.
Classification
Ventricular septal defect in newborns and older children can be diagnosed as an independent problem (isolated defect) or as a component of other cardiovascular diseases, for example, Cantrell's pentad (click here to read about it).
The size of the defect is estimated based on its size in relation to the diameter of the aortic opening:
- a defect up to 1 cm in size is classified as small (Tolochinov-Roger disease);
- Large defects are considered to be defects from 1 cm or those that in size exceed half of the aortic mouth.
Finally, according to the location of the hole in the septum, VSD is divided into three types:
- Muscular ventricular septal defect in a newborn. The hole is located in the muscular part, away from the conduction system of the heart and valves, and if small in size, can close on its own.
- Membranous. The defect is localized in the upper segment of the septum below the aortic valve. Usually it has a small diameter and stops on its own as the child grows.
- Supracrest. It is considered the most complex type of defect, since the hole in this case is located on the border of the efferent vessels of the left and right ventricles, and very rarely closes spontaneously.
What drugs can be used in the treatment of atrial septal defect?
For postoperative patients, antiplatelet and anticoagulant medications, such as aspirin or warfarin (Coumadin), are usually prescribed for three to six months after the procedure. Because the occluder is a foreign object, this treatment is necessary to prevent blood clots from forming around the device.
Diuretics ( diuretics ) are prescribed when symptoms of heart failure and fluid retention and swelling are detected. Diuretics promote the release of excess fluid and salts. Since potassium loss is possible during diuretic therapy, potassium-containing drugs (Asparkam) are prescribed in combination with diuretics. To increase the contractile activity of the heart, digoxin is prescribed, which is also able to reduce the heart rate, remove excess fluid from tissues and has a hypotensive effect in case of high blood pressure.
Treatment
Includes three tasks. Relieving symptoms, eliminating the defect itself, and preventing potentially fatal complications. Everything is decided at the same moment.
With a small IVS defect, regardless of the patient’s age, if there are no significant disturbances, arrhythmias, or other symptoms, and the condition does not progress, a wait-and-see approach is chosen.
Every few months, objective indicators are assessed; if there is a negative course, surgery is indicated.
It is carried out as planned
Before surgery, it is important to prepare the patient and stabilize his condition with medication.
The specific names of the drugs depend on the age of the patient and the level of functional impairment.
As a rule, the following remedies are indicated:
- Antihypertensive. They are limited to beta blockers to relieve high blood pressure and eliminate tachycardia.
- Cardioprotectors. Restore metabolism in the heart.
- Medicines based on potassium and magnesium. Nourishes the myocardium and normalizes contractility.
The duration of the preparatory period is about 2-3 months, more is extremely rare.
The surgical intervention itself consists of suturing the defect (with dissection of the chest), or restoring the anatomical integrity of the tissues using an occluder (without dissection of the sternum).
The rehabilitation period lasts approximately six months. There are no significant restrictions in later life. If the pathology is eliminated, everything returns to normal.
How should nutritional deficiencies be compensated for in children with ASD?
Newborns and children with ASD always gain weight very slowly, and the most common cause of such poor growth is excess heat energy production or insufficient dietary energy intake. Other factors that often cause impaired physical development and excessive energy expenditure in children with ASD include the following:
- increased heart rate and increased breathing rate
- poor appetite
- frequent respiratory infections
- decreased absorption of nutrients by the digestive tract
- decreased blood oxygen saturation
One of the features of newborn nutrition is that a child with an ASD quickly becomes overtired during feeding. Pediatricians recommend feeding the baby every two hours in small portions in the first two months after birth and feeding should be done only on demand. In some newborns, malnutrition may be associated with the design of the feeding bottle, so parents must choose the most suitable option for the child. If the child is prescribed medications, it is better to take them before feeding and it is important that the drug and breast milk or formula are not mixed. The pediatrician will also need to inform parents about the timing of the transition to solid food, approximately 6 months after birth. It is advisable to avoid eating foods containing fats for 2 years. The use of balanced high-calorie nutrient mixtures is considered optimal for rapid growth. When forming a diet for children over 2 years of age, it is recommended to adhere to the following nutritional principles:
- Fat intake should be less than 30% of total calories consumed during the day
- the number of calories from saturated fat should be no more than 8-10% of the total calories consumed per day
- cholesterol intake should not exceed 300 mg/dl per day
A consistent transition to the use of the so-called Mediterranean diet will further prevent the earlier development of coronary heart disease in adult life. Foods should primarily consist of grains, vegetables, fruits, lean meats and other foods that are low in fat and high in complex carbohydrates and proteins. It is advisable to exclude salt when preparing dishes, and also avoid eating salty foods. To do this, it is necessary to exclude from the diet fast food products, canned foods and frozen foods, in which salt is used to increase shelf life.
How does the postoperative period proceed after surgery to close an atrial septal defect?
Children who undergo open ASD surgery or endovascular closure usually require lifelong monitoring and monitoring by a pediatric and then adult cardiologist. Along with standard examinations, these patients require ongoing monitoring of their heart condition. Usually, regular diagnostics of the heart condition are carried out before and after surgery, and later, if the results are positive, parents forget about the need for constant monitoring, which is not entirely correct. It is important to constantly undergo preventive examinations, do follow-up studies and maintain close contact with your doctor, surgeon and cardiologist. In addition, to prevent the occurrence of various infections associated with the weakening of the body's immunity after surgery, immunization, for example, immunization with a flu vaccine .
What is the prognosis after surgery for atrial septal defect?
Prospects and prognoses for achieving good results in the treatment of ASD have improved significantly over the past two decades. At the moment, patients with a small defect can live a normal life with minimal recommendations, but as mentioned earlier, patients with large ASDs require surgical treatment. Performing endovascular closure is accompanied by very good results; when performing reconstructive open operations, the results are somewhat worse, but to a greater extent they are due to the treatment of already advanced forms of the disease and the presence of complex reconstructions. Currently, less than 1% of patients with ASD who undergo complex reconstructive interventions do not live to see their 45th birthday. It is also statistically estimated that approximately 5-10% of patients operated on over the age of 40 years die from complications of surgery, these figures once again highlight the need for early detection and treatment of atrial septal defect. Despite the figures announced, it should be recalled once again that without surgical treatment, almost a quarter (25%) of patients with ASD die. As practice shows, treatment of ASD before the age of 20 practically guarantees the return of the child to a full and normal life.