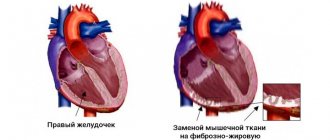
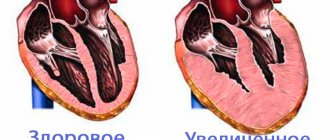
Pathology of the cardiac myocardium, in which expansion of the heart chambers occurs, is a dangerous disease fraught with serious consequences for the entire body. The disease is called dilated cardiomyopathy. The expansion of the heart chamber does not lead to its compaction.
The worsening of the disease is due to the fact that the ventricles stretch and lose their ability to contract. The heart muscle is depleted too quickly in this position.
The disease is fraught with sudden death of the patient. The age group of patients with dilated cardiomyopathy is from 20 to 55 years, in most cases it occurs in men.
What it is
Dilated cardiomyopathy of the heart (ICD code 10 - international classification of diseases), a collective name for various diseases and pathological processes in the human body that lead to disruption of the cardiac myocardium. The nature of these diseases and phenomena is not clear.
Diagnosis of the disease is complicated by the absence of pronounced symptoms, since the body is still able to compensate for the disruption of the myocardium and heart muscle.
ICD 10 myocardial dystrophy – Information portal “Your Heart”
Included: acute pericardial effusion
Excludes: rheumatic pericarditis (acute) (I01.0)
Excluded:
- some current complications of acute myocardial infarction (I23.-)
- postcardiotonic syndrome (I97.0)
- heart injury (S26.-)
- diseases specified as rheumatic (I09.2)
Excluded:
- acute rheumatic endocarditis (I01.1)
- endocarditis NOS (I38)
Excluded: mitral (valvular):
- disease (I05.9)
- deficiency (I05.8)
- stenosis (I05.0)
for an unknown reason, but mentioning it
- aortic valve disease (I08.0)
- mitral stenosis or obstruction (I05.0)
lesions specified as congenital (Q23.2-Q23.9)
lesions specified as rheumatic (I05.-)
Excluded:
- hypertrophic subaortic stenosis (I42.1)
- for unknown cause, but with mention of mitral valve disease (I08.0)
- lesions specified as congenital (Q23.0, Q23.1, Q23.4-Q23.9)
- lesions specified as rheumatic (I06.-)
Excluded:
- without specifying the reason (I07.-)
- lesions specified as congenital (Q22.4, Q22.8, Q22.9)
- specified as rheumatic (I07.-)
Excluded:
- lesions specified as congenital (Q22.1, Q22.2, Q22.3)
- disorders specified as rheumatic (I09.8)
Endocarditis (chronic) NOS
Excluded:
- endocardial fibroelastosis (I42.4)
- cases specified as rheumatic (I09.1)
- congenital aortic valve insufficiency NOS (Q24.8)
- congenital aortic valve stenosis NOS (Q24.8)
Included: endocardial damage due to:
- candida infection (B37.6)
- gonococcal infection (A54.8)
- Libman-Sachs disease (M32.1)
- meningococcal infection (A39.5)
- rheumatoid arthritis (M05.3)
- syphilis (A52.0)
- tuberculosis (A18.8)
- typhoid fever (A01.0)
Excluded:
- cardiomyopathy, complicating: pregnancy (O99.4)
- postpartum period (O90.3)
Excluded:
- cardiogenic shock (R57.0)
- complicating: abortion, ectopic or molar pregnancy (O00-O07, O08.8)
- obstetric surgical interventions and procedures (O75.4)
Excluded:
- complicating: abortion, ectopic or molar pregnancy (O00-O07, O08.8)
- obstetric surgical interventions and procedures (O75.4)
- NOS (R00.0)
Excluded:
- bradycardia: NOS (R00.1)
- sinoatrial (R00.1)
- sinus (R00.1)
- vagal (R00.1)
- abortion, ectopic or molar pregnancy (O00-O07, O08.8)
Excluded:
- conditions complicating: abortion, ectopic or molar pregnancy (O00-O07, O08.8)
- obstetric surgical interventions and procedures (O75.4)
- kidney disease (I13.-)
Excluded:
- any conditions listed in sections I51.4-I51.9 due to hypertension (I11.-)
- any conditions listed in sections I51.4-I51.9 due to hypertension with renal disease (I13.-)
Excludes: cardiovascular disorders NOS in diseases classified elsewhere (I98*)
In Russia, the International Classification of Diseases, 10th revision (ICD-10) has been adopted as a single normative document for recording morbidity, reasons for the population's visits to medical institutions of all departments, and causes of death.
ICD-10 was introduced into healthcare practice throughout the Russian Federation in 1999 by order of the Russian Ministry of Health dated May 27, 1997. No. 170
The release of the new revision (ICD-11) is planned by WHO in 2022.
International classification and definition of myocardial dystrophy
If the patient has myocardial dystrophy, it will be difficult to assign a code according to ICD-10 in this case. Since the last revision, a separate code is no longer allocated for this pathology. However, there is a code that is used from the section on cardiomyopathy.
Myocardial dystrophy is understood as a specific heart disease that is not inflammatory in nature. It is characterized by metabolic disorders that occur in myocytes. At the same time, the contractility of the heart muscle changes, and heart failure appears.
Classification of diseases ICD-10 is a normative document that contains codes for diseases systematized by sections. This is an international development that facilitates the collection and storage of information to create statistics for all countries. ICD-10 was created by the World Health Organization.
The document is revised every 10 years and additions are always made to it. If previously there was a separate code for myocardial dystrophy, now it is not assigned to this disease according to ICD-10. However, doctors can put the number I.
42, which implies cardiomyopathy.
Despite the similarity of names, cardiomyopathy and myocardial dystrophy are considered different phenomena, since the first involves a wider range of different cardiac pathologies that are not associated with heart disease and the vascular system.
In general, section I42 assumes cardiomyopathy, but excludes conditions that are complicated by pregnancy and postpartum ischemic disease. Number I42.0 suggests a dilated type, and 42.1 is a hypertrophic obstructive pathology. Number 42.2 refers to other forms of hypertrophic pathology. Code 42.
3 is endomyocardial disease, and 42.4 is fibroelastosis of the endocardial type. If the patient has other restrictive cardiac pathologies, then the number is set to 42.5.
If cardiomyopathy is of alcoholic origin, then code 42.6 is assumed. If the disease is caused by the influence of drugs or other external factors, then the number 42.7 is set. Other cardiomyopathies are numbered 42.
8, and if the forms of pathology are not specified, then code 42.9.
Brief description of the disease
Myocardial dystrophy is a disease that disrupts the normal functioning of the myocardium and leads to degeneration of the heart muscle. The danger of this pathology is that it interferes with normal metabolism.
If left untreated, myocardial dystrophy in children and adults leads to atrial fibrillation and severe heart failure, and in some situations – to the development of chronic heart diseases, myocarditis and cardiomyopathies.
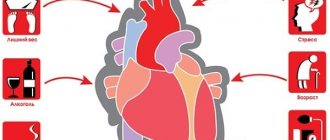
Factors contributing to the occurrence of myocardial dystrophy are divided into two groups - cardiac and extracardiac. The first include heart defects that cause metabolic disorders in the heart muscle. In turn, extracardiac causes are associated with blood diseases, vitamin deficiencies, intoxications, radiation and diseases of the endocrine glands.
In addition, myocardial dystrophy of the heart can manifest itself as a result of occupational hazards, exposure to physical factors (overheating, chest trauma), emotional and physical stress. The progression of the above reasons leads to disruption of metabolic processes in myocardial tissues.
What happens with myocardial dystrophy?
The dystrophic process has 3 stages of development:
- the first stage - individual foci of unfavorable changes appear, which lead to the death of myocardial tissue and an increase in its size. Over time, isolated lesions merge into one whole, forming expanded cavities of the heart. At this stage, myocardial dystrophy is easily treatable, since initial disturbances in the contractile function of the heart can be easily relieved with the help of medications;
- second stage – the pathological process progresses, the affected area spreads to healthy tissue. In this case, the death of muscle cells is compensated by increasing the volume of undamaged structures, however, dystrophic changes are still reversible due to the normalization of muscle fibers and their intracellular restoration;
- the third stage - advanced myocardial dystrophy - symptoms indicate the development of heart failure and atrial fibrillation, which, in turn, cause irreversible changes in the myocardium and lead to serious complications in the functioning of the cardiovascular system.
Myocardial dystrophy - symptoms of the disease
In some cases, determining the correct cause of the development of the pathology and prescribing adequate treatment is hampered by the fact that the signs of myocardial dystrophy are often masked as symptoms of the underlying disease. Patients experience:
- increased fatigue with minor physical exertion;
- dyspnea;
- moderate tachycardia;
- muffled heart sounds;
- pain in the left side of the chest;
- in the later stages of development - signs of heart failure.
The task of doctors is to correctly interpret the clinical symptoms of the dystrophic process, excluding the development of cardiomyopathies, myocarditis and coronary heart disease.
Myocardial dystrophy - treatment of the disease
Treatment of myocardial dystrophy is based on eliminating the cause of the pathology, that is, the factor that caused the dystrophic changes. In this case, we are talking about surgical and drug treatment of endocrine diseases, removal of foci of chronic infections, prevention of intoxication, and reduction of the degree of negative impact of occupational hazards.
In addition, dyshormonal myocardial dystrophy requires adherence to a strict diet. The patient should increase the proportion of foods containing large amounts of protein and vitamins in the diet. If you are overweight, the calorie content of dishes is reduced, and if you have vitamin deficiency and dystrophy, they increase it.
The most important therapeutic direction for myocardial dystrophy is the correction of electrolyte disturbances. Patients are prescribed intravenous administration of 1 g of potassium chloride or 10-15 mg of panangin diluted in a 5% glucose solution. If the patient has mild myocardial dystrophy, the symptoms of which are mild, potassium preparations can be prescribed orally.
In the treatment of all forms of myocardial dystrophy, agents that stimulate myocardial metabolic processes are also used. These drugs include: B vitamins, riboxin, potassium orotate, anabolic steroids. If myocardial dystrophy leads to the appearance of symptoms of heart failure, cardiac glycosides are indicated for patients.
We also note that, regardless of the form and stage, the treatment of myocardial dystrophy must be comprehensive.
Activities are carried out over a long period of time until electrolyte disturbances disappear and ECG readings normalize.
After discharge from the hospital, all former patients are subject to mandatory follow-up with a cardiologist. If necessary, the doctor will determine a set of further treatment measures and the composition of rehabilitation procedures.
As for the prevention of the disease, it is aimed at timely prevention of those processes that lead to dystrophy of the heart muscle.
Myocardial dystrophy in children
At an early age, heart pathologies arise due to rickets, hypervitaminosis, chronic nutritional disorders, bacterial diseases and viral infections. In addition, myocardial dystrophy in children can develop due to physical overexertion or, conversely, due to the child’s low physical activity.
As a rule, at an early age the symptoms of pathology are not clearly expressed. For this reason, you should immediately consult a doctor at the first suspicion of heart problems. To diagnose myocardial dystrophy, standard measures in such cases are carried out, namely: a thorough examination by a cardiologist, an electrocardiogram, and an ultrasound of the heart.
Source: //znk-mos.ru/vtorichnaya-miokardiodistrophiya-kod-po-mkb-10/
Clinical picture
The first symptoms of dilated cardiomyopathy in children and adults appear only with a decrease in myofibrils - cell structures, the presence of which ensures the contractile function of the heart muscle. At the same time, the metabolism of metabolic substances inside cells begins to decrease.
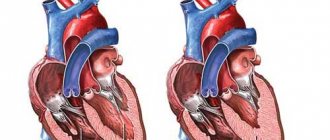
With the development of cardiomyopathy with dilatation of the heart cavities in the first stages, the organ is able to maintain its functional duties at a normal level.
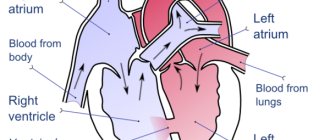
Dilation of the heart chambers
In the future, the disease can cause the development of serious complications:
- Increased myocardial volume.
- Disturbance of the heart valves.
- Reducing the volume of blood that enters the aorta from systole.
- Development of chronic heart failure.
- Lack of oxygen in the heart muscle.
- Circulatory disorders.
All of the above abnormalities in the functioning of the heart lead to disorders of the central nervous and endocrine systems. Increased production of hormones has an extremely negative effect on the biological components of the blood. Blood thickening occurs, which in turn leads to the formation of numerous blood clots that clog the blood vessels.
Without a timely diagnosis and proper treatment, the heart muscle will completely weaken, lead to degeneration of internal organs, and sudden death will occur.
Causes
Etiology • Many HCM are hereditary diseases resulting from mutations in genes encoding myocardial contractile proteins. Familial hypertrophic cardiomyopathy: • type 1: 192600, MYH7, CMH1, 160760 (cardiac myosin, heavy chain b7), 14q12; • type 2: 115195, TNNT2, CMH2, 191045 (cardiac troponin 2), 1q32; • type 3: 115196, TPM1, CMH3, 191010 (tropomyosin cardiac 1), 15q22; •; type 4: 115197, MYBPC, CMH4, 600958 (myosin binding protein C), 11p11.2; • type 7: TNNI3, 191044 (cardiac troponin I), 19p13.2 q13.2; • with Wolff Parkinson-White syndrome: CMH6, 600858, 7q3
Pathogenesis. As a result of gene mutations, left ventricular hypertrophy and areas of cardiomyocyte disorganization occur.
• An increase in the content of calcium ions in cardiomyocytes and pathological stimulation of the sympathetic nervous system are important.
• Abnormally thickened intramural arteries do not have the ability to adequately dilate, which leads to ischemia, myocardial fibrosis and pathological hypertrophy.
• With asymmetric hypertrophy of the interventricular septum, according to the latest data, the obstruction is associated mainly with the abnormal forward movement of the anterior mitral valve leaflet into systole and, to a lesser extent, with septal hypertrophy (obstruction of the outflow tract of the left ventricle - muscular subaortic stenosis: the left ventricle is “divided” into two parts: a relatively small subaortic and a large apical; during the period of expulsion, a pressure difference occurs between them).
• Due to the presence of obstructions to normal blood flow, the pressure gradient between the left ventricle and the aorta increases, which leads to an increase in end-diastolic pressure in the left ventricle. Most patients have abnormal left ventricular systolic function.
• Regardless of the pressure gradient between the left ventricle and the aorta, patients with HCM have impaired left ventricular diastolic function, leading to increased end-diastolic pressure, increased pulmonary capillary wedge pressure, pulmonary congestion, left atrial dilatation, and atrial fibrillation. The development of diastolic dysfunction is associated with a decrease in compliance and impaired relaxation of the left ventricle •• Decreased compliance occurs due to increased muscle mass, a decrease in the left ventricular cavity and a decrease in myocardial compliance due to its fibrosis •• Deterioration of relaxation is the result of systolic (incomplete emptying of the left ventricle due to outflow tract obstruction) and diastolic (decreased ventricular filling) disorders.
• HCM in some cases is accompanied by myocardial ischemia, which is associated with the following reasons •• Reduced vasodilator reserve of the coronary arteries •• Abnormal structure of the intramural arteries of the heart •• Increased myocardial oxygen demand (increased muscle mass) •• Compression during systole of the arteries passing through the thicker myocardium •• Increased diastolic filling pressure •• In addition to the above reasons, 15–20% of patients experience concomitant atherosclerosis of the coronary arteries.
Pathomorphology • Macroscopic examination •• The main morphological manifestation of HCM is thickening of the walls of the left ventricle over 30 mm (sometimes up to 60 mm) in combination with normal or reduced dimensions of its cavity •• Dilation of the left atrium (arises due to increased end-diastolic pressure in the left ventricle ) •• In most patients, the interventricular septum and most of the lateral wall of the left ventricle are hypertrophied, while the posterior wall is less often involved in the process. In other patients, only the interventricular septum hypertrophies. In 30% of patients there may be local hypertrophy of the wall of the left ventricle of small sizes: the apex of the left ventricle (apical), only the posterior wall, the anterolateral wall. In 30% of patients, the right ventricle, papillary muscles or apex of the heart are involved in the hypertrophic process • Microscopic examination •• Disorderly arrangement of cardiomyocytes, replacement of muscle tissue with fibrous tissue, abnormal intramural coronary arteries •• The presence of disordered hypertrophy, characterized by multidirectional arrangement of myofibrils and unusual connections between neighboring myocardial cells •• Foci of fibrosis are represented by randomly intertwined bundles of coarse collagen fibers.
Factors causing the development of the disease
Disease of the heart muscle, in which myocardial depletion is noted, is divided into two types, depending on the reasons that prompted its development - primary and secondary cardiomyopathy.
The primary form includes myocardial disorders, the nature of which is unknown.
Doctors can only speculate on the possible causes of dilated cardiomyopathy:
- Hereditary factor. If a person has relatives in their family who have had serious heart problems, there is a high risk that cardiomyopathy will be genetically transmitted.
- Diseases of the autoimmune type, which provoke the development of autoimmune changes in myocarditis (hemorrhagic vasculitis, glomerulonephritis, arthritis).
The secondary type of cardiomyopathy occurs due to various pathologies, the nature of which has been studied by medicine:
- Arterial hypertension.
- Diseases of viral origin - herpes, influenza.
- Intoxication of the body with alcoholic beverages and narcotic substances.
- Excessive smoking.
- Heart valve disease.
- Myocarditis.
- Myocardial ischemia.
- The use of highly targeted medications that are used to treat cancer.
- Hormonal imbalance (with a strict diet, fasting, vitamin deficiency).
- Diseases of the endocrine system - all types of diabetes, pathological processes in the adrenal glands, disorders in the thyroid gland.
- Excessive emotional and physical stress.
All these reasons may not lead to the development of myocardial pathologies. But if a person has a hereditary factor, he is at risk. If there is a genetic predisposition, the prognosis for the future is extremely unfavorable, especially if the person leads an unhealthy lifestyle and has bad habits.
International classification and definition of myocardial dystrophy
Other cardiac pathologies
06.10.2016
19.8 thousand
13.3 thousand
4 min.
If the patient has myocardial dystrophy, it will be difficult to assign a code according to ICD-10 in this case. Since the last revision, a separate code is no longer allocated for this pathology. However, there is a code that is used from the section on cardiomyopathy.
- 1. ICD-10 code
- 2. Forms of the disease
- 3. Causes and symptoms
Myocardial dystrophy is understood as a specific heart disease that is not inflammatory in nature. It is characterized by metabolic disorders that occur in myocytes. At the same time, the contractility of the heart muscle changes, and heart failure appears.
Classification of diseases ICD-10 is a normative document that contains codes for diseases systematized by sections. This is an international development that facilitates the collection and storage of information to create statistics for all countries. ICD-10 was created by the World Health Organization.
The document is revised every 10 years and additions are always made to it. If previously there was a separate code for myocardial dystrophy, now it is not assigned to this disease according to ICD-10. However, doctors may give the number I.42, which implies cardiomyopathy.
Despite the similarity of names, cardiomyopathy and myocardial dystrophy are considered different phenomena, since the first involves a wider range of different cardiac pathologies that are not associated with heart disease and the vascular system. Therefore, these terms cannot be used as synonyms.
But for myocardial dystrophy there is still one code according to ICD-10, which assumes only cardiomyopathy with metabolic disorders and nutritional problems.
In general, section I42 assumes cardiomyopathy, but excludes conditions that are complicated by pregnancy and postpartum ischemic disease. Number I42.0 suggests a dilated type, and 42.1 is a hypertrophic obstructive pathology.
Number 42.2 refers to other forms of hypertrophic pathology. Code 42.3 is an endomyocardial disease, and 42.4 is fibroelastosis of the endocardial type. If the patient has other restrictive cardiac pathologies, then the number is set to 42.5.
If cardiomyopathy is of alcoholic origin, then code 42.6 is assumed. If the disease is caused by the influence of drugs or other external factors, then the number 42.7 is set. Other cardiomyopathies are numbered 42.
8, and if the forms of pathology are not specified, then code 42.9.
There are several forms of myocardial dystrophy. Depending on the type of dystrophic processes in the heart, the following are known:
- restrictive;
- dilatational;
- hypertrophic.
Depending on the severity of the disease, the following forms are distinguished:
- 1. Compensation. Hemodynamics are still maintained at the level required for the body.
- 2. Subcompensation. The mechanisms that are responsible for hemodynamics are strained, even with moderate physical activity. There is already pronounced dystrophy of the heart muscle.
- 3. Decompensation. With little physical activity, deviations in hemodynamics are noticeable. The contractile capabilities of the myocardium are sharply weakened.
Depending on the pathogenesis, primary and secondary forms are distinguished. The primary one assumes that the cause of the disease has not been established. Secondary means that myocardial dystrophy appeared as a complication against the background of another disease.
Depending on the disease that caused dystrophy of the heart muscle, the following forms are distinguished:
- 1. Dishormonal. Appears due to disturbances in the synthesis of sex hormones. A person often gets tired, has problems sleeping, is thirsty, and loses weight. A stabbing or aching pain is felt in the area of the heart.
- 2. Tonsillogenic. It is a complication after tonsillitis. Endurance deteriorates, aching pain in the heart area, and arrhythmia appear.
- 3. Alcoholic. Develops as a consequence of prolonged alcohol intoxication. The membranes of cardiac tissue cells are destroyed due to exposure to ethanol. Potassium and fatty acid reserves decrease. This leads to shortness of breath and arrhythmia. Pain in the heart area almost does not appear.
- 4. Anemic. Often appears in pregnant women. At later stages, toxicosis also appears.
- 5. Diabetic. Appears in patients with type 1 diabetes mellitus. In this case, diabetic disorders can be detected in the coronary vessels, and angina pectoris develops.
The occurrence of myocardial dystrophy as a primary disease is quite rare. Usually this pathology is secondary. All causes of myocardial dystrophy can be divided into 2 groups. The first includes all the heart ones.
For example, this could be cardiomyopathy or myocarditis. The second group includes non-cardiac causes.
These are problems with metabolic processes, anemia, intoxication of the body, diseases caused by infections, and exposure to adverse environmental factors (radiation, weightlessness, overheating, etc.).
Because of this, the myocardial muscles do not receive enough nutrients and oxygen and experience intoxication. Gradually the cells die and are replaced by connective tissue. Scars appear. As a result, the main functions of the myocardium are disrupted - contraction, conductivity, excitability and automaticity. Because of this, all cells of the human body suffer.
At the initial stage of the disease, symptoms do not appear, so the disease does not make itself felt.
But if the disease is not treated, heart failure gradually develops, which can even lead to death. Therefore, as soon as the first signs of illness appear, you need to urgently go to the hospital. The reason for this is the following:
- shortness of breath with minor physical exertion;
- strong increase in heart rate at low loads;
- weakness, a person gets tired quickly;
- pain and discomfort in the chest area;
- cough in the evening and at night with a large amount of sputum.
Symptoms of the disease, depending on its form and causes, may be different. If they are detected, you should immediately consult a doctor for diagnosis and selection of appropriate therapy.
Myocardial dystrophy is a specific non-inflammatory disease. With this disease, metabolic disorders are observed due to the action of various factors. Since the last revision, there is no separate code for this syndrome in ICD-10, so the code must be selected from section I42, which describes the types of cardiomyopathy.
Source: //vashflebolog.com/other-heart-diseases/miocardiodistrophiya-kod-po-mkb-10.html
Symptoms
At the first stage of the disease, there are no external symptoms. Only an echocardiogram can show any changes. The clinical picture manifests itself during the period of disruption of the circulatory process and the formation of stagnation in the blood vessels.
Main symptoms of cardiomyopathy:
- Symptoms of shortness of breath. At first, breathing problems appear only after physical exertion, later shortness of breath begins to torment in a passive state, and the extreme degree appears when a person takes a horizontal position.
- Swelling of the legs.
- Asthma, pulmonary edema and other respiratory disorders. As a rule, a wet cough occurs along with suffocation; blood streaks are released in the sputum. During attacks of suffocation, the patient experiences acrocyanosis of the skin - blue discoloration of the upper and lower extremities, lips, and tip of the nose. Asthmatic attacks occur, in most cases, during night sleep.
- Chronic fatigue.
- Fast fatiguability.
- Pain in the liver area.
- Attacks of rapid heartbeat (cardiac tachycardia).
- Fainting conditions.
- Dizziness.
- Problems with urination. The patient may feel the need to go to the toilet too often, and vice versa.
- Insomnia.
- Memory impairment.
- Frequent, causeless changes in mood.
- Pain in the chest area.
Frequent chest pain may be a sign of dilated cardiomyopathy
When cardiomyopathy is at an advanced stage, the patient begins to use a sitting position during sleep to reduce shortness of breath.
The abdomen always enlarges, which is caused by the accumulation of a large amount of fluid in the abdominal cavity. Shortness of breath appears at the slightest physical activity. When listening to the lungs, a gurgling sound is noted.
Clinic
The similarity of the clinical signs of dyshormonal cardiomyopathy with other diseases of the heart and blood vessels is very pronounced. In particular, with DHCM, heart pain similar to false angina is often observed, which makes it difficult to make a correct diagnosis.
Pain in the heart in dyshormonal cardiomyopathy is localized at its apex and is characterized by a varying area of distribution - from small (in the left shoulder) to extensive (covers the entire left half of the chest). It has varying intensity (from aching to strong and unbearable).
Characteristics of emotional coloring of pain:
- "hit";
- "piercing nail"
- “piercing long needles”;
- “The heart is filled with boiling water.”
In every third case (32.6%), a combination of dishormonal cardiomyopathy with initial manifestations of coronary heart disease (combined forms of the disease) is observed.
Heart pain syndrome in such cases is complicated due to the combination of false angina with true angina.
IHD, which occurs in patients at a later age (after about 50 years) compared to dyshormonal cardiomyopathy (47 years) and in most cases in postmenopausal women.
“Hot flashes” in patients with menopausal cardiopathy, in contrast to patients with typical forms, the symptoms of menopause are less pronounced, although their frequency of occurrence is usually less than 10 per day.
In addition to cardialgia and “hot flashes,” other signs may be detected in patients with DHCM:
- arrhythmia;
- heartbeat;
- dizziness;
- feeling of lack of air;
- severe autonomic disorder;
- fluctuations in blood pressure;
- general neuroticism of the patient.
The following can be considered “harbingers” of DHCM in women:
- Late onset of menarche (average 14.5 years)
- Inhibition of reproductive function (approximately 3 pregnancies and a 1:2 birth to abortion ratio)
- A large number of gynecological diseases (uterine fibroids, chronic inflammatory processes of the internal genitalia, dysfunctional uterine bleeding)
- Early onset of menopause (average age 46.5 years).
Men tolerate the development of DHCM not so brightly. As a rule, the pathological process affects the prostate gland, so patients often note frequent urination, alternating with the urge.
In some cases, on the contrary, oliguria is observed (a decrease in the amount of daily urine), which may be accompanied by decreased libido.
Such disorders often cause erectile dysfunction, another sign of DHCM in men.
Diagnostics and therapeutic methods
To establish a diagnosis, a series of medical tests are performed and the patient's medical history is studied. Blood sampling is done for a series of tests - biochemical, immunological and coagulogram. The patient needs to undergo an x-ray, phonocardiogram, echocardiogram.
Conservative methods are used to treat cardiomyopathy; in particularly severe cases, surgery is performed. The patient needs to mentally prepare for the fact that taking medications will be lifelong. Medicines from the group of inhibitors, adrenoblockers, diuretics, and cardiac glycosides are prescribed.
Surgery for cardiomyopathy is recommended only in extreme cases, since this type of surgery is extremely difficult, and many patients die during it.
Modern cardiac surgery offers the following surgical methods for the treatment of cardiomyopathy:
- Installation of a pacemaker.
- Valve prosthesis.
- Implantation of a mechanical pump for pumping blood.
- Installation of an extracardiac frame.
If the operation is successful, the survival rate is very high. Despite this, such surgical interventions are not widespread due to the very high cost and long waiting lists for donor organs.
Diagnosis of alcoholic cardiomyopathy
On examination, stigmata of chronic alcohol intoxication are revealed, as well as facial hyperemia, moist skin, acrocyanosis, large-scale tremors of the hands, edema of the lower extremities, and ascites. Vesicular or harsh breathing is heard in the lungs, and congestive moist rales are heard in the lower parts. In the initial stage of the disease, a moderate expansion of the borders of the heart, tachycardia at light loads, weakening of the first sound at the apex, and an increase in systolic and diastolic pressure are noted.
As the disease progresses, a significant expansion of the borders of the heart in both directions, tachycardia at rest, a pronounced weakening of the first tone, and a gallop rhythm are revealed. Heart sounds may be arrhythmic due to atrial fibrillation, and a systolic murmur may be heard at the apex. Palpation of the abdomen can reveal an enlarged liver, which is usually painless, soft or somewhat dense in consistency, with a rounded edge.
Increased serum activity of GGT, AST and ALT.
The ECG reveals sinus tachycardia, atrial and ventricular extrasystole, paroxysms of atrial fibrillation. Also characteristic are changes in the final part of the ventricular complex in the form of a pointed T wave, which can subsequently decrease and become smoothed. Subsequently, atrial fibrillation becomes constant, and signs of left ventricular myocardial hypertrophy may appear.
Echocardiography (EchoCG) determines an increase in the end-systolic and end-diastolic size of first the left ventricle, and then other chambers of the heart, the end-diastolic pressure in the left ventricle increases, and the ejection fraction decreases. With an advanced process, there is a significant expansion of all chambers of the heart and a decrease in the thickness of the walls of the left ventricular myocardium.
Differential diagnosis is carried out with myocarditis, post-infarction cardiosclerosis, heart defects.
On examination, stigmata of chronic alcohol intoxication are revealed, as well as facial hyperemia, moist skin, acrocyanosis, large-scale tremors of the hands, edema of the lower extremities, and ascites. Vesicular or harsh breathing is heard in the lungs, and congestive moist rales are heard in the lower parts. In the initial stage of the disease, a moderate expansion of the borders of the heart, tachycardia at light loads, weakening of the first sound at the apex, and an increase in systolic and diastolic pressure are noted.
As the disease progresses, a significant expansion of the borders of the heart in both directions, tachycardia at rest, a pronounced weakening of the first tone, and a gallop rhythm are revealed. Heart sounds may be arrhythmic due to atrial fibrillation, and a systolic murmur may be heard at the apex. Palpation of the abdomen can reveal an enlarged liver, which is usually painless, soft or somewhat dense in consistency, with a rounded edge.
[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]
Considering that the leading clinical syndrome in dilated cardiomyopathy is heart failure, treatment should be based on the prescription of ACE inhibitors and diuretics. ACE inhibitors not only increase the left ventricular ejection fraction, increase patients' tolerance to physical activity and, in some cases, improve the functional class of circulatory failure, but also improve the prognosis of life, reduce mortality, and increase survival in patients with low ejection fraction.
According to some data, beta-blockers improve the prognosis and general condition of the patient. It is recommended to start treatment with small doses. Drugs from the group of beta-blockers, affecting the hyperactivation of the sympathoadrenal system, have shown the ability to improve hemodynamics and the course of heart failure, have a protective effect on cardiomyocytes, reduce tachycardia and prevent rhythm disturbances.
Treatment of heart failure should be carried out in accordance with the National Guidelines for the diagnosis and treatment of CHF.
Malignant ventricular arrhythmias are the leading cause of sudden cardiac death in patients with dilated cardiomyopathy. However, in patients with an advanced form of the disease, the causes of up to 50% of cases of cardiac arrest may be bradyarrhythmias, embolism of the pulmonary artery and other vessels, and electromechanical dissociation.
- sustained ventricular tachycardia (class I evidence);
- syncope (class I evidence);
- decreased left ventricular ejection fraction (class IIa evidence);
- unsustained ventricular tachycardia (class IIB evidence);
- induction of ventricular tachycardia during electrophysiological study (class III evidence).
For sinus tachycardia, symptomatic treatment is carried out with beta-blockers or verapamil, starting with minimal doses.
Patients with ventricular extrasystole have an increased risk of sudden death, however, antiarrhythmic drugs do not improve the prognosis in asymptomatic cases with a diagnosis of DCM or in the presence of only palpitations. In case of symptoms of left ventricular failure, beta-blockers are added to treatment. For high-grade ventricular extrasystole, amiodarone, sotalol, and class Ia antiarrhythmic drugs are used.
In the presence of ventricular tachycardia and hemodynamically significant disorders (fainting, presyncope, arterial hypotension), an unfavorable prognosis of the disease should be assumed. It is recommended to prescribe treatment with amiodarone, which reduces mortality by 10-19% in patients with a high risk of sudden death, and the need for implantation of a cardioverter or defibrillator should also be considered. In patients with sustained ventricular tachycardia and dilated cardiomyopathy when heart transplantation is not possible, the main treatment method is implantation of a cardioverter or defibrillator.
The choice of method for stopping paroxysm of ventricular tachycardia is determined by the state of hemodynamics: if it is unstable, then synchronized cardioversion is performed (discharge power 200 J). If hemodynamics are stable, intravenous administration of lidocaine (bolus continuous infusion) is recommended.
For atrial fibrillation, treatment tactics depend on its form (paroxysmal, persistent, permanent). Thus, with the development of paroxysmal atrial fibrillation and the presence of a rapid ventricular rhythm, signs of heart failure that do not quickly respond to pharmacological agents, immediate electrical cardioversion is indicated.
Medical or electrical cardioversion to quickly restore sinus rhythm is indicated in patients with a new episode of atrial fibrillation. In patients with cardiomegaly, i.e. DCM, restoration of sinus rhythm in a permanent form of atrial fibrillation is contraindicated. If drug or electrical cardioversion is ineffective, ventricular rate control is carried out in combination with antithrombotic treatment [indicated in the case of atrial fibrillation and impaired left ventricular function (presence of chronic heart failure, left ventricular ejection fraction less than 35%)], To control heart rate with a permanent form of atrial fibrillation, a combination of cardiac glycosides and beta-blockers is more effective.
Surgical treatment of dilated cardiomyopathy (heart transplantation, cardiomyoplasty, use of an artificial left ventricle) is indicated when medication is ineffective, but is rarely performed, mainly in young and middle-aged patients.
Heart transplantation is indicated for progressively worsening heart failure and if DCM develops in a patient under 60 years of age.
The main alternative to heart transplantation today is the use of circulatory support devices, which are called artificial ventricles.
- Left ventricular ejection fraction {amp}lt;45% and/or shortening fraction {amp}lt;25% assessed by echocardiography, radionuclide scanning or angiography.
- Left ventricular end-diastolic size {amp}gt;117% of predicted value, adjusted for age and body surface area.
- Criteria for excluding the diagnosis of DCM.
- Systemic hypertension ({amp}gt;160/100 mmHg).
- Atherosclerotic lesion of the coronary arteries (stenosis {amp}gt;50% in one or more large branches).
- Alcohol abuse ({amp}gt;40 g/day for women and {amp}gt;80 g/day for men for more than 5 years after 6 months of abstinence).
- A systemic disease that could lead to the development of dilated cardiomyopathy.
- Pericardial diseases.
- Congenital and acquired heart defects.
- Pulmonary heart.
- Confirmed accelerated supraventricular tachycardia.
Patients typically describe having various symptoms of heart failure that have worsened over the past few months or years. Symptoms may appear before cardiomegaly is detected by echocardiography and chest x-ray. It is necessary to actively clarify the fact of alcohol abuse, as it may play a role in the progression of primary dilated cardiomyopathy.
- X-ray of the chest organs
Cardiomyopathy in children
Cardiomyopathy in children can be congenital, or acquired as a result of injuries during childbirth, or if the mother suffered severe infectious diseases during pregnancy. Treatment of cardiomyopathy in a child is identical to that in adult patients.
The use of traditional medicine methods in the treatment of cardiomyopathy has no effect. Taking decoctions of medicinal herbs - calendula, chamomile or motherwort, you can help improve the general condition of the body, but will not have an effect on the heart ventricles.