Even with proper medical treatment, mortality from heart failure remains high. Most clinical trials did not include patients with severe or end-stage heart failure (stage D). These patients are often indicated for heart transplantation.
However, in 2001 in the United States, 40,000 patients were on the waiting list for heart transplantation, while only 2,102 were performed. Alternatives to heart transplantation are needed to close this gap. Many surgical interventions are being developed to at least improve the condition of patients and allow them to survive until heart transplantation. Improvements in cardiac surgery and a better understanding of the changes that occur in heart failure have led to the proliferation of several new surgical treatment methods. However, there is still insufficient data on the safety and effectiveness of many methods. This article discusses elective operations for heart failure (myocardial revascularization, mitral valve surgery, cardiomyoplasty, geometric reconstruction of the left ventricle - Dora operation) and emergency circulatory support measures (up to a total artificial heart). Surgical interventions must be accompanied by active drug treatment. Coronary artery bypass grafting for ischemic cardiomyopathy. Mitral valve surgeries Reconstructive surgeries on the left ventricle Assisted circulation
Coronary artery bypass grafting for ischemic cardiomyopathy
With severe damage to the coronary arteries, the blood supply to the myocardium suffers and cardiomyocytes are exposed to hypoxia, as a result of which their work is impaired. With myocardial infarction, necrosis occurs, and then scars are formed that are unable to contract. The areas of the myocardium adjacent to the infarction are subject to mechanical stretching, as a result of which, over time, the left ventricle undergoes restructuring, its cavity increases, and systolic and diastolic function worsens. Severe ischemia, in addition to myocardial infarction, can cause its stunning and transition to a state of “hibernation” (sleeping myocardium). At the same time, cardiomyocytes can remain viable and, when their blood supply is restored, restore their function. Sleepy and stunned myocardium can be detected using special methods.
Myocardial stunning is the loss of contractility due to short-term acute ischemia. Dormant myocardium occurs during chronic ischemia; loss of contractility allows it to maintain viability. The sleeping myocardium continues to take up glucose from the blood, but the mass of contractile proteins in it decreases and glycogen accumulates.
Clinical significance
Often, especially with initial cardiac damage, ischemia may manifest itself not as angina, but as heart failure. In approximately two thirds of cases, the main cause of impaired contractility is damage to the coronary arteries. Coronary angiography is indicated in all cases where there is a suspicion of an ischemic origin of dilated cardiomyopathy. Sometimes damage to the coronary arteries is superimposed on dilated cardiomyopathy of a different etiology. In this case, the degree of contractility impairment does not correspond to the severity of damage to the coronary arteries. In these patients, the feasibility of revascularization is questionable.
Recommendations
There are no reliable data on the effectiveness of coronary artery bypass grafting for ischemic cardiomyopathy. However, according to clinical observations and case-control studies, with proper selection of patients, coronary artery bypass grafting improves the prognosis for ischemic cardiomyopathy. Therefore, when the ejection fraction of the left ventricle is more than 15%, the end-diastolic size of the left ventricle is less than 65 mm, the distal bed of the coronary arteries is suitable for bypass surgery and a large amount of ischemic or asleep myocardium, coronary bypass surgery is indicated. These recommendations are conditional; in many clinics, coronary bypass surgery makes patients even sicker. However, those who require continuous IV inotropic infusion do not usually undergo coronary artery bypass grafting. In cases of severe left ventricular systolic dysfunction and the presence of quiescent myocardium, coronary artery bypass grafting can be as effective as heart transplantation (three-year survival rate is approximately 80%).
It is generally believed that significant improvement justifying surgery is possible if the proportion of myocardium that is asleep and ischemic, but working, is more than 60%. Perioperative mortality is increased if more than 40% of the left ventricle is scar tissue or nonviable (metabolically inactive) myocardium.
Coronary artery bypass grafting for severe heart failure is part of a comprehensive treatment that may also include the following: valve repair, geometric reconstruction of the left ventricle (Dora procedure), destruction of the sources of ventricular tachycardia and labyrinthine surgery (Cox procedure) or pulmonary vein isolation for atrial fibrillation. To achieve maximum effect, it is necessary to continue active drug treatment after surgery.
Treatment of heart failure, medications used for treatment
Today there are many more treatments for heart failure than in the past.
Primary treatment for heart failure usually involves taking medications regularly, maintaining a healthy lifestyle, and closely monitoring your condition. If the disease progresses, the patient may require surgical treatment of heart failure.
What medications are used to treat congestive heart failure?
Strict adherence to the schedule and dosage of medications recommended by your doctor is an important condition for maintaining your own health. Most often prescribed for the treatment of heart failure:
- Angiotensin-converting enzymes (ACE);
- Angiotensin II receptor blockers (ARBs);
- Beta blockers;
- Digoxin;
- Diuretics;
- Blood vessel dilators;
- Potassium or magnesium;
- Calcium channel blockers;
- Medicines to support heart function.
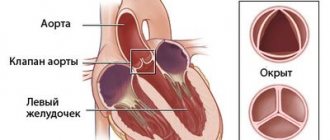
Mitral valve surgery
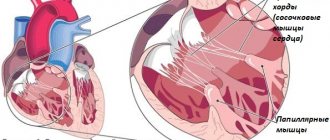
Regardless of the cause of left ventricular dysfunction, its dilation and change in shape lead to mitral regurgitation. This in turn leads to volume overload of the left ventricle, further dilatation and further worsens mitral regurgitation. Contributions to mitral regurgitation include damage to the valve itself and its ring, ischemia and infarction of the papillary muscles, changes in the shape of the left ventricle, thinning of the myocardium and dilatation of the left ventricle, divergence of the papillary muscles and valve leaflets with disruption of their closure.
Clinical significance of mitral regurgitation and recommendations
- Restoring valve leaflet closure by annuloplasty reduces mitral regurgitation and improves the shape of the left ventricle; this may increase cardiac output in dilated cardiomyopathy. In ischemic cardiomyopathy, mitral valve repair is, however, less effective than in cases of damage to the valve itself.
- Subvalvular structures are kept intact whenever possible.
- Mitral valve repair improves well-being in some patients, but it is unclear whether it affects survival.
- Alfieri's mitral valve approximation sometimes produces more reliable results than simple annuloplasty.
- In most cases, mitral valve replacement is not required; the prognosis after it is much worse than after plastic surgery.
How to prevent heart failure from getting worse?
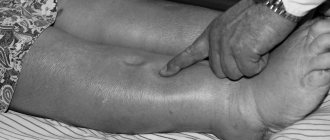
To prevent congestive heart failure from progressing, it is important to monitor your health very closely. Do not violate the recommended medication regimen. And you need to regularly visit your doctor to make sure that the disease is not developing. Fluid retention in the body is one of the symptoms of heart failure and is a reason to immediately consult a doctor. Dizziness, weakness, shortness of breath can also be signs of heart failure, and if these symptoms reappear during treatment, do not delay a visit to a specialist.
Reconstructive operations on the left ventricle
According to Laplace's law, when the heart chamber dilates, the stress in its wall increases in proportion to the radius of the chamber. At the same time, the myocardial oxygen demand increases and the processes of pathological restructuring of the myocardium intensify. Surgical plastic surgery of the ventricle makes it possible to reduce its cavity and thereby reduce the tension in its wall. Geometric reconstruction of the left ventricle with an intraventricular patch (Dora operation) is performed for ischemic cardiomyopathy with a large area of akinesia or dyskinesia of the left ventricular myocardium. Resection of the left ventricle (Batista procedure) was previously performed for dilated cardiomyopathy of non-ischemic origin; however, despite the promising immediate effect, the long-term results of this operation were very poor.
Geometric reconstruction of the left ventricle using an intraventricular patch (Dora operation)
After myocardial infarction, a scar forms and restructuring of the left ventricle occurs, leading to its dilatation and the development of heart failure. For scars and an aneurysm in the area of the anterior descending artery with relatively normal contractility of the lateral and posterior segments of the left ventricle, it is possible to perform the Dora operation. The area of akinesia or dyskinesia is opened, a purse-string suture is placed at the base of the aneurysm and tightened. The remaining hole is closed with a Dacron patch, after which the ventriculotomy hole is sutured with a running suture. In more than 90% of cases, coronary bypass surgery is performed simultaneously with the Dora operation. In addition, mitral valve plastic surgery and destruction of arrhythmic foci are often performed. Operation Dora is indicated for patients with an aneurysm of the apex of the left ventricle (or a large area of akinesia), a high index of end-systolic volume of the left ventricle, the absence of scar changes in the area of the circumflex artery and viable myocardium in the area of planned coronary artery bypass grafting. During the operation, the severity of mitral insufficiency is assessed and in 30-50% of cases, mitral valve repair is performed. After surgery, the ejection fraction of the left ventricle increases, the end-diastolic and end-systolic volumes of the left ventricle decrease, the shape of the left ventricle becomes closer to normal, and the severity of heart failure decreases. In a number of observations, survival after this operation in patients with severe heart failure was 98% after 1 year, 95.8% after 2 years, and 82.1% after 5 years. Unfavorable prognostic factors: high functional class of heart failure before surgery, low ventricular ejection fraction (<20%), high index of left ventricular end-systolic volume, other areas of impaired contractility in addition to the apex.
Left ventricular resection (Batista procedure)
Resection of the left ventricle (Batista operation) consists of excision of the myocardium of the lateral wall of the left ventricle between the papillary muscles; at the same time, plastic surgery or replacement of the mitral valve is possible. The operation was used for dilated cardiomyopathy of non-ischemic origin. As already mentioned, this operation is not currently carried out.
Cardiomyoplasty
The idea of the operation is to force the skeletal muscle to work to help the myocardium or instead of it. It turned out that quickly fatigued fibers (intermediate and white) under the influence of electrical stimulation can turn into slowly fatigued (red) fibers, such as in the myocardium. The operation consists of mobilizing the latissimus dorsi muscle and moving it through the hole created by resection of the second rib into the chest cavity. Then, using a longitudinal sternotomy approach, the muscle is wrapped around the heart and secured posteriorly next to the right atrium and pulmonary artery, and anteriorly around the right ventricle. The sensing electrodes are placed on the epicardium of the right ventricle, and the stimulating electrodes are placed in the thickness of the latissimus dorsi muscle. Within 2 weeks after the operation, the muscle adapts to stimulation, while impulses are supplied with every second contraction of the heart. In the next 12 weeks. impulses are slightly increased every 1-2 weeks. The effect of cardiomyoplasty is due, firstly, to increased contractions of the left ventricle, and secondly, to the scaffolding effect of the muscle flap. This prevents left ventricular dilatation and improves well-being, but there is no evidence of improved survival after cardiomyoplasty. Currently, cardiomyoplasty is rarely used, and long-term results are unknown.
Other surgical methods
Cardiac casing (ACORN). This device is a medical fabric sheath that fits over the heart and adheres to the epicardium without causing significant fibrosis or constriction. The cardiac casing, which is sometimes supplemented with mitral valve plastic, reduces tension in the wall of the left ventricle and prevents its overdistension in diastole and further dilatation. Heart tie (Myosplint). This device is a mechanical tie that penetrates the ventricle and is secured on the epicardial side with two plates. The distance between the plates is adjustable, which allows you to bring the walls of the left ventricle closer to each other. As a result, the ventricle is divided into two lobes of smaller radius. By reducing the radius, the tension in the wall of the left ventricle is reduced. However, implantation of such a tie is fraught with aggravation of mitral insufficiency; in this case, mitral valve repair is required. Stem cell transplantation is performed to regenerate the myocardium shortly after myocardial infarction. It is believed that stem cells can form a functioning myocardium. How much this is actually possible is not clear. The technique has not been properly developed, and the optimal timing of the procedure has not been established. The procedure may increase the risk of arrhythmias. Concomitant drug treatment has not been developed.
Balanced diet
Many patients are frightened by the very word diet, so it is better to use the term “rational nutrition.” In heart failure, it has two main features. The first is salt restriction.
At the 1st stage, it is necessary to avoid salty foods, at the second it is forbidden to add more salt, at the third stage special dietary products with a reduced percentage of NaCl are used. The second feature is the limit on liquid consumption (up to 1.5 liters (including first courses) per day). The general principle of nutrition for people with pathology - food rich in vitamins, animal proteins, with a reduced cholesterol content - is also applicable in this case.
Assisted circulation
Assisted circulatory support is necessary in cases of severe hemodynamic impairment, when the prognosis without heart transplantation is extremely unfavorable. Assisted circulation helps keep a patient alive while waiting for a heart transplant. Assisted circulation includes intra-aortic balloon counterpulsation, extracorporeal membrane oxygenation, ventricular and biventricular permanent or pulsatile pumps (artificial ventricles), artificial heart. The choice of one or another method is determined by the expected duration of use, the reversibility of the cause of cardiogenic shock, the need to maintain the function of one left or both ventricles, as well as the patient’s physique. Circulatory support is usually used when, despite intensive drug treatment, hemodynamics remain unstable. Most often these are patients who need a heart transplant.
Systolic blood pressure is less than 75-80 mm Hg. Art. Cardiac index less than 1.5-1.8 l/min/m2. Blood oxygen saturation in mixed venous blood (SvO2) is less than 50%.
Circulatory support may be used short-term for cardiogenic shock in the following situations.
- After heart surgery
- For myocardial infarction
- For fulminant myocarditis
- When circulatory arrest occurs during cardiac surgery (survival rate in this case is low)
If it is expected that the pumping function of the heart will soon be restored, it is best to choose the least complex and traumatic method of assisted circulation. For small patients (with a body surface of less than 1.3 m2), only external devices with centrifugal pumps are suitable. If restoration of the pumping function of the heart is not expected, long-term implantable devices must be used.
Devices for short-term circulatory support
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation is a method of extracorporeal circulation in which blood is sucked out by a centrifugal pump and enters a membrane oxygenator, where carbon dioxide is exchanged for oxygen. Blood is taken from the femoral vein and returned to the femoral artery. Extracorporeal membrane oxygenation requires systemic anticoagulant therapy and may cause significant damage to blood components. This method allows you to saturate the blood with oxygen in case of severe respiratory failure. In addition, the right ventricle is unloaded.
Category pump Hemopump
The Hemopump category pump can only be used for a few days. It, like the intra-aortic balloon pump, is inserted through a catheter in the femoral artery. However, unlike intra-aortic balloon counterpulsation, which only partially facilitates the work of the left ventricle, the catheter pump completely takes over it. The catheter is passed through the aortic valve into the left ventricle. The catheter with a diameter of 14, 21 or 24 F contains a permanent pump, the productivity of which reaches 3.5-5.7 l/min. Anticoagulant therapy, constant monitoring and bed rest are necessary. In addition, the catheter pump does not unload the right ventricle and cannot be used in cases of damage to the aorta and aortic valve. The most common complications are hemolysis and ventricular arrhythmias caused by contact of the catheter with the myocardium.
Centrifugal pumps
Centrifugal pumps are used extracorporeally. They are usually used to support both ventricles in patients with a body surface area of less than 1.3 m2. Typically, centrifugal pumps are combined with extracorporeal membrane oxygenation. The flow in these pumps is non-pulsating; it is formed by the rotation of impellers or funnel-shaped impellers. Blood is taken from the right atrium and returned to the aorta. Catheters are inserted through a sternotomy access; usually only the skin is sutured. The operation of the device requires constant monitoring; long-term use is not possible. Heparin therapy is required.