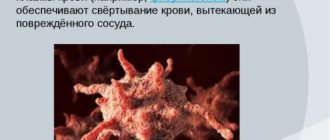
Activated platelet on glass with immobilized fibrinogen.
Scanning electron microscopy. Platelets
(from the Greek θρόμβος - clot and κύτος - cell; outdated name - blood platelets) are small (2-4 microns) anucleate flat colorless blood cells formed from megakaryocytes.
Functions
Scanning electron micrograph (SEM) of human blood cells: red blood cell, activated platelet, white blood cell (from left to right).
Platelets perform two main functions:
- Formation of a platelet aggregate, a primary plug closing the site of vessel damage;
- Providing its surface to accelerate key plasma coagulation reactions.
Relatively recently, it was found that platelets also play a critical role in the healing and regeneration of damaged tissues, releasing growth factors into damaged tissues that stimulate cell division and growth. Growth factors are polypeptide molecules of various structures and purposes. The most important growth factors include platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF)[ 1].
The physiological plasma concentration of platelets is 180-360*10^9 platelets per liter.
A decrease in the number of platelets in the blood can lead to bleeding. An increase in their number leads to the formation of blood clots (thrombosis), which can block blood vessels and lead to pathological conditions such as stroke, myocardial infarction, pulmonary embolism, or blockage of blood vessels in other organs of the body.
A deficiency or disease of platelets is called thrombocytopathy, which can be either a decrease in the number of platelets (thrombocytopenia), a violation of the functional activity of platelets (thrombasthenia), or an increase in the number of platelets (thrombocytosis). There are diseases that reduce platelet counts, such as heparin-induced thrombocytopenia or thrombotic purpura, which usually cause thrombosis instead of bleeding.
Due to the inaccuracy of descriptions, the lack of photographic equipment and the confusing terminology of the early periods of the development of microscopy, the exact time of the first observation of platelets is unknown. Most often, their discovery is attributed to Donna (1842, Paris), but there is evidence that they were observed by the creator of the microscope himself, Antonie van Leeuwenhoek (1677, the Netherlands). The term "blood platelets", which is still preferred in the English-language literature (blood platelets), was introduced by Bizzozero (1881, Turin), who also played a leading role in identifying the connection of platelets with homeostasis and thrombosis. This subsequently led to the emergence of the term “platelet” (Dekhuizen, 1901), which became the main one in the Russian language. In the English-language literature, the term is used exclusively for nucleated platelets in non-mammalians (thrombocytes). In addition, in Russian literature the term “Bizzocero plaque” may be used for platelets.
Description of blood cells
The structural components of blood are divided into 2 fractions - red and white. Platelets are classified as red. They do not have nuclei and are synthesized by the bone marrow from megakaryocytes, large cells with a standard structure (volumetric nuclei).
In a calm state, if there is no vascular damage, the red blood cells are flat and resemble circles or ovals in appearance. They can only be seen under a microscope; their sizes are 2-5 microns. As soon as the integrity of the capillaries, arteries or veins is compromised, the shape of the platelets changes - they increase in size and swell.
By the appearance of the structural components of physiological fluid, you can understand what is happening in the body:
- Calm state - no changes. 90% of cells are fully mature.
- With massive blood loss, under a microscope you can see a lot of large platelets on a glass slide; the bone marrow is intensively producing new cells.
- When hematopoiesis is disrupted, anucleate cells are small and degenerative.
- Slow development and uneven shapes suggest a malignant process.
- A sharp increase in size indicates diseases of the hematopoietic system.
The lifespan of platelets is 8 days. They are then destroyed in the spleen, bone marrow and liver. During normal life, renewal is continuous. Characteristics by size: megaforms, macroforms, microforms and normoforms.
Participation in folding
A special feature of the platelet is its ability to activate - a rapid and, as a rule, irreversible transition to a new state. The activation stimulus can be almost any environmental disturbance, even simple mechanical stress. However, the main physiological activators of platelets are collagen (the main protein of the extracellular matrix), thrombin (the main protein of the plasma coagulation system), ADP (adenosine diphosphate, appearing from destroyed vascular cells or secreted by the platelets themselves) and thromboxane A2 (a secondary activator synthesized and released by platelets; its additional function is to stimulate vasoconstriction).
Activated platelets become able to attach to the site of damage (adhesion) and to each other (aggregation), forming a plug that covers the damage. In addition, they participate in plasma coagulation in two main ways - exposure of the procoagulant membrane and secretion of α-granules.
Exposure of the procoagulant membrane
Under normal conditions, the platelet membrane does not support clotting reactions. Negatively charged phospholipids, primarily phosphatidylserine, are concentrated in the inner layer of the membrane, while phosphatidylcholine in the outer layer binds coagulation factors much less well. Although some coagulation factors can bind to non-activated platelets, this does not lead to the formation of active enzymatic complexes. Activation of the platelet presumably leads to the activation of the scramblase enzyme, which begins to quickly, specifically, bidirectionally and ATP-independently transfer negatively charged phospholipids from one layer to another. As a result, thermodynamic equilibrium is established, in which the concentration of phosphatidylserine in both layers is equalized. In addition, upon activation, many transmembrane proteins in the outer layer of the membrane undergo alignment and/or conformational changes, and they acquire the ability to specifically bind coagulation factors, accelerating reactions involving them.
Platelet activation has several degrees, and procoagulant surface expression is one of the highest. Only thrombin or collagen can produce such a strong response. A weaker activator, especially ADP, can contribute to the work of stronger activators. However, they are not able to independently cause the appearance of phosphatidylserine; their effects are limited to changes in platelet shape, aggregation and partial secretion.
Secretion of α-granules
Platelets contain several types of granules, the contents of which are secreted during activation. Essential for coagulation are α-granules containing high molecular weight proteins such as factor V and fibrinogen.
Features of the structure of platelets
The dimensions of nuclear-free plates are microscopic, up to 5 microns. But each of them includes several layers responsible for certain functions. Thanks to this structure, cells quickly respond to unfavorable organic changes. In addition to microtubules and granules, which contain substances necessary for blood clotting, individual platelets include ribosomes, non-membrane organelles. They are responsible for the contractility of the vascular walls.
Peripheral zone
The outer layer is a 50 nm thick membrane consisting of 3 layers. It contains plasma factors, enzymes and receptors that regulate aggregation - the ability of cells to stick together. The membrane forms spongy folds that penetrate the cell lengthwise and crosswise, which allows it to penetrate into the deepest layers of all tissues and organs.
The membrane contains phospholipase, a component of arachidonic acid. This substance is necessary for the production of prostaglandins. They, in turn, stimulate the production of thromboxane, which is necessary to increase the rate of aggregation (sticking together) and the formation of coagulation complexes.
Glycoproteins
These compounds are found in the lipid biolayer of the membrane. Function: adhesion of flat cells, binding to protein (fibronectin). The clot that forms on the surface of the wound contracts, becomes stronger and decreases in size. If there are not enough glycoproteins, the wound will bleed for a long time.
Sol-gel
Responsible for cell contraction and increased aggregation ability. This zone is located in the submembrane layer and consists of a ring of microtubules and protrusions. ADP (adenosine triphosphate, responsible for metabolic processes at the cellular level) and ATP (adenosine triphosphoric acid, a source of intracellular energy), calcium, serotonin, and anti-clotting (antiheparin) factor accumulate here.
When an impulse is transmitted from the brain, the ring of microtubules contracts, clotting factors are released into the lumen of blood vessels, the blood thickens, the ability to aggregate increases and pseudopodia protrude. The process is called granule centralization.
Thrombocytopenia is a disease in which the level of red squamous blood cells decreases (the lower level can drop to 109 m/l). The first sign is increased bleeding, a blood clotting disorder. If you notice these symptoms, you should consult a doctor. The danger of the condition is a high risk of internal bleeding.
Intracellular organelles
The organelle zone contains: dense bodies, mitochondria, glycogen granules, α-granules. Dense bodies include, as already mentioned, ADP and ATP, calcium and the hormones adrenaline, norepinephrine and serotonin. When the process of centralization of granules occurs, microtubules release these substances into the blood:
- calcium - increases the intensity of adhesion;
- ADP and ATP - normalizes the adhesion function;
- hormones - narrows the lumens of blood vessels.
Tests to evaluate the vascular-platelet component of hemostasis
- Bleeding time;
- The number of platelets in the blood;
- Induced platelet aggregation.
Qualitative platelet defects, which underlie a large number of hemorrhagic diathesis, are divided into the following groups:
- disaggregation thrombocytopathies caused by the absence or blockade of platelet membrane receptors (Glanzmann’s thrombasthenia, etc.);
- diseases of the absence of dense and α-granules;
- granule release disorders;
- disturbances in the formation of cyclic prostaglandins and thromboxane A2;
- deficiency, anomalies and disorders of the multidimensionality of von Willebrand factor;
- disorders of nucleotide metabolism and calcium transport.
Norm
Platelet standards for adults and children (*10 9 /l):
- children; newborns – 100 – 420;
- from 2 weeks to a year – 150 – 350;
- from one year to 5 years – 180 – 380;
- from 5 years to 7 years – 180 – 450;
- 180 – 320;
The content of platelets in the blood depends on the time of day and season of the year. Physiological daily fluctuations in the number of blood platelets are about 10%. Cyclic changes in the amount of PLT population in women during menstruation can reach up to 25–50%.
This change in platelets in a blood test in women of reproductive age reaches its maximum increase immediately after menstruation, which is typical for any other blood loss, and the minimum PLT value is the level of this population in the second half of the monthly cycle.