Norms of electrolytes in the blood in adults and children
Blood is a multicomponent biological fluid that performs many key functions for the body. Each of its components has its own role and normal concentration.
Let's take a closer look at such a substance as electrolytes - should they be present in the blood and in what quantity? How to properly donate blood for electrolytes and what options are there for deciphering the test results?
Electrolytes - what is this in a biochemical blood test?
Electrolytes are breakdown products of acid, salt and alkaline compounds. The blood contains electrolytes with different discharges:
- Anions are negatively charged (phosphates, bicarbonates, chlorides and acids of organic origin).
- Cations are positively charged (particles of calcium, magnesium, sodium, potassium).
Electrolytic substances enter the body with food. Metabolized by the liver and kidneys.
Of all blood components, electrolytes account for about 1% of the total composition; these substances can be found both inside and outside the cell.
Due to their qualitative and quantitative diversity, electrolytes perform several important functions:
- regulate the level of conductivity in membranes and cell excitability;
- catalyze thrombus formation during injuries and bleeding;
- control blood clotting;
- regulate blood pH balance;
- participate in bone formation;
- activate most enzymes;
- maintain homeostasis (stable state of the body regardless of external factors);
- transport fluid from the blood to other tissues (regulation of water balance);
- promote the removal of decay products from the cell;
- maintain normal conduction of nerve impulses.
Each electrolytic element performs its own task in the body. The most important compounds for humans are chloride, potassium and sodium compounds.
- Potassium promotes the removal of toxins, prevents oxygen starvation of tissues, stimulates the heart and its rhythm, supports the protective function and prevents the development of allergic reactions.
- Sodium activates many substances and hormones, regulates transport and thereby allows the body to develop and grow.
- Chlorine works in tandem with sodium, it keeps the water-salt balance under control and prevents its disturbance.
Indications for a blood test for electrolytes
A change in electrolyte balance is one of the signs of chronic or acute pathologies in the body.
An analysis of electrolyte concentrations is used as a diagnostic measure in case of suspected diseases of the cardiovascular system, metabolic disorders and the presence of some specific symptoms:
- arrhythmias of various nature;
- arterial hypertension;
- liver and pancreas diseases;
- kidney disorders;
- multiple burns;
- unspecified illnesses accompanied by psycho-emotional disorders, swelling, headaches and dizziness, prolonged nausea and other gastrointestinal symptoms, heavy blood loss, diarrhea.
A blood test for electrolytes is also used to monitor the progress of the disease and the effectiveness of treatment.
Standards for electrolyte content in children and adults
In patients of different sexes, the norms differ only in the concentration of two elements:
- iron (Fe): for men - 17.8 - 22.5, for women - 14.5 - 17.8;
- phosphorus (Ph): for men - 1.86 - 1.45, for women - 0.8 - 1.32.
Important! The norm is individual for each person; it is determined by the general state of health, age and other physiological indicators.
The normal content of electrolytes Mg, Ca and Cl in children and adults is the same.
For other substances the threshold is as follows:
- K (potassium): in children under one year old - 4 - 5.4, over one year old - 3.4 - 5.5;
- iron (Fe): in children under one year old - 6 - 19, over one year old - 8 - 22;
- phosphorus (Ph) - not related to age, the general norm is 1.18 - 2.79.
A decrease or increase in the amount of electrolytes in the blood can lead to an imbalance in the overall water balance. Because of this, metabolic processes are inhibited and the functions of almost all organs are disrupted.
Table
The concentration of electrolytes in blood plasma is measured in mmol/liter. In adult men and women, the norms for the content of individual electrolytes are practically the same.
ElectrolyteNormal
| Cl (chlorine) | 97 — 107 |
| Ca (calcium) | 2,15 — 2,5 |
| K (potassium) | 3,5 — 5,5 |
| Mg (magnesium) | 0,65 — 1,05 |
| Na (sodium) | 136 — 146 |
Basic rules for donating blood for electrolytes
The concentration of electrolytes is highly dependent on the influence of external factors.
In order for the result of the study to be as accurate as possible, you need to prepare for the test.
Preparation includes several basic rules:
- Avoid physical and emotional stress in the last 48 hours.
- It is recommended to stop taking medications a few days before the test (if possible).
- The day before visiting the laboratory, avoid strong tea and coffee drinks, too spicy or salty foods.
- Donate blood in the morning and on an empty stomach (the last meal should be no later than 8 hours before the test).
- Before taking blood, do not drink anything or smoke (!).
Before you go into the office and donate blood, it is better to just sit and calm down.
Analysis methodology
The material for studying the concentration of electrolytes is venous blood .
To determine the number of compounds, the laboratory technician uses one of the research methods:
- Weight method. Enzymes are added to the blood sample. They react with plasma components resulting in a precipitate. This precipitate is separated and weighed. Then the mass fraction of each blood component is determined.
- Atomic spectral method. The biomaterial is heated to high temperatures. Then, based on molecular spectral analysis (a linear absorption spectrum is used), the qualitative and quantitative composition of the sample is determined.
- Photoelectrocolorimetry. The blood is placed in a sterile tube to which a special solution is added. A reaction occurs at the end of which the contents of the test tube turn a certain color. The shade is compared with a special table from which the concentration of electrolytes is determined.
- Express method. A special analyzer is used, which almost instantly determines the concentration of electrolytic compounds and the acid-base balance of the blood plasma.
Upon completion of the analysis, the results obtained are recorded in a special form. This document is sent to the attending physician, who interprets the analysis and makes a diagnosis.
Decoding the research results
Electrolyte imbalances most often occur in people who have illnesses or poor diets.
Due to an incorrectly formulated diet, the amount of electrolytic compounds entering the blood plasma may pathologically decrease or, on the contrary, increase.
Young and elderly patients are very sensitive to such disorders, since their compensatory mechanisms have either not yet developed well enough, or, on the contrary, do not work well due to age.
Reduced levels of electrolytes in the blood
A decrease in the amount of each electrolyte is characterized by separate pathologies.
A decrease in potassium levels indicates:
- excessive physical activity;
- stress and nervous tension;
- alcoholism;
- abuse of coffee drinks and sweets;
- dyspepsia;
- unbalanced diet;
- cystic fibrosis;
- hyperhidrosis.
The main symptoms of hypokalemia are shortness of breath, irregular heart rhythm, heart pain, impaired reflexes and convulsions, general malaise, and exhaustion.
Hyponatremia usually develops with the following disorders:
- cirrhosis;
- insufficient salt intake;
- dehydration;
- persistent increase in body temperature;
- lack of thyroid hormones;
- heart and kidney diseases;
- hyperglycemia;
- nephrotic syndrome.
With this disorder, patients suffer from hypotension, psychoemotional disorders, loss of appetite, vomiting and nausea.
The main reasons for reducing the amount of chlorine electrolytes:
- increased sweating;
- acidotic coma;
- traumatic brain injury;
- disorders in the gastrointestinal tract.
With hypochloremia, people actively lose hair and their teeth deteriorate.
The following pathologies can be the causes of hypocalcemia:
- fragility and inhibition of bone growth;
- hypothyroidism;
- pathology of the pancreas;
- bile stagnation;
- renal dysfunction and liver disorders;
- severe exhaustion of the body;
- abuse of antiepileptic drugs and cytostatics.
Hypoconcentration of phosphorus, iron and magnesium most often appears due to:
| Magnesium | Phosphorus | Iron |
|
|
|
Note! The main reason for the decrease in the concentration of all electrolytes is the uncontrolled use of diuretics and an unbalanced diet.
Increased levels of electrolytes in the blood
Hyperconcentration develops against the background of the following phenomena:
HyperkalemiaHypernatremiaHyperchloremiaHypercalcemiaHypermagnesemiaElevated iron levelsHyperphosphatemia
| starvation; | oversaturation with salt; | alkalosis; | lack of parathyroid hormones; | lack of thyroid hormones; | lead poisoning; | hormone and chemotherapy; |
| disturbance of nerve conduction; | hormone therapy; | dehydration; | bone cancer; | dehydration; | anemia of various nature; | therapy with diuretics and antiseptics; |
| erythrocyte hemolysis; | tumors in the adrenal glands; | excess adrenal hormones; | poisoning with thyroid hormones; | abuse of drugs with magnesium; | violation of hemoglobin synthesis; | renal pathologies; |
| dehydration; | camatoses; | lack of vasopressin. | spinal tuberculosis; | diseases of the kidneys and adrenal glands. | blood diseases; | tumor growth and metastasis; |
| hyperacidity; | pituitary hyperplasia; | gout; | hemochromatosis. | increased lipid concentration; | ||
| adrenal diseases; | disorders in the endocrine system. | excess vitamin D and insulin. | diabetic ketoacidosis; | |||
| excess potassium; | lack of thyroid parahormones; | |||||
| long-term therapy with non-steroidal anti-inflammatory drugs and cytostatics. | excess growth hormone; |
Price for laboratory analysis
The price for a biochemical blood test can range from 500 to 1000 rubles , depending on the specific clinic. The study is carried out in almost every laboratory, including SYNEVO, CMD and Invitro.
Source: https://normatela.com/krov/elektrolity-analiz-norma
Decoding of the Invitro analysis
The clinic’s medical staff will help you correctly interpret the results when you contact the laboratory or the company’s hotline (8-800-200-363-0). Or go to the clinic’s personal account.
Go to your Invitro personal account
- To find out the result of Invitro testing online, click the “Login” button;
- “Results without registration”;
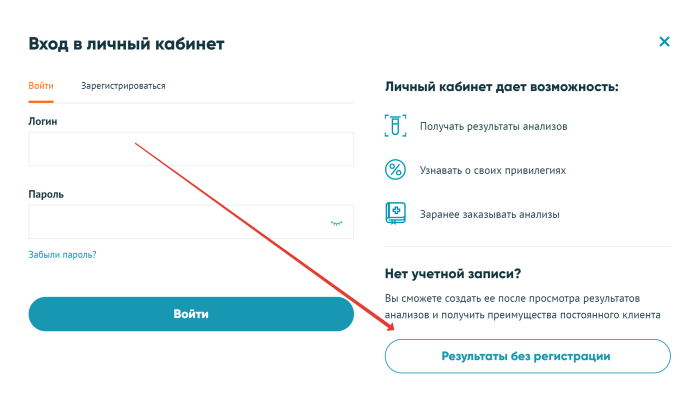
- To see the test results, fill in the fields, select the method for obtaining the result and click the “Find Results” button.
Analysis of blood electrolytes: indications, preparation, norms, treatment
Electrolytes are always present in the blood. These are substances formed from salts, alkalis and acids, which decompose to form cations and anions. During a biochemical blood test, it is mandatory to determine the electrolyte level. Their deviation from the norm leads to severe health problems and poses a threat to the patient’s life.
What are electrolytes?
Electrolytes occupy an important place in the biochemical analysis of blood
Electrolytes in the blood are positively or negatively contaminated particles formed during the breakdown of salts, acids and alkalis in the blood during natural physiological processes. The main electrolytes in humans are:
- magnesium,
- potassium,
- iron,
- sodium,
- calcium,
- chlorine,
- phosphorus.
Particles are present in blood plasma and are involved in most processes occurring in human tissues and organs. There are many reasons for electrolyte imbalance. Some of them are associated with serious pathologies, which is why, if deviations are detected during analysis, an examination is required.
Functions and role of electrolytes
One of the functions of electrolytes is to ensure impulse transmission
https://www.youtube.com/watch?v=RuA0uXdZJfQ
Electrolytes are present in the blood and intercellular space, entering it through cell membranes. The particles normalize the transfer of fluid from the blood to the cells of tissues and organs, and also maintain the correct acidity in the blood and ensure the full passage of nerve impulses.
Depending on the element, electrolytes perform different functions. Thus, they help maintain proper functioning of the heart muscle, bone formation, blood clotting, and metabolic processes. Deviations from the norm have a negative impact on the entire body.
Indications for electrolyte analysis
Heart rhythm disturbances - indication for analysis
A blood test for the volume of electrolytes is carried out according to medical indications. The main reasons for the study are:
- examination to diagnose a disease when the patient has dizziness, behavioral disturbances, and nausea;
- arrhythmias;
- comprehensive measures for diagnosing liver and pancreas diseases;
- determination of the most effective drugs for a particular patient with hypertension.
Also, the analysis can be prescribed according to medical prescriptions for pregnant women and people suffering from chronic pathologies of the gastrointestinal tract and heart.
Preparing for the study
The study is carried out on an empty stomach
Preparation for analysis allows you to obtain the most accurate research result. The main points in it are:
- refusal to eat 12 hours before blood donation;
- You can drink only clean water without gas in the morning before the analysis;
- exclusion of physical and emotional stress the day before the analysis;
- quit smoking 2 hours before the test.
When taking medications, the doctor must be warned about this so that the specialist, when interpreting the results, can make adjustments for the effect of the drugs.
Methods for determining the amount of electrolytes
Electrolyte levels are determined using a biochemical blood test. One of two methods can be used.
- Weight. It is based on carrying out certain chemical reactions in which blood serum is used. As a result of these actions, a precipitate is obtained that does not dissolve in water. It is weighed on special scales. Next, the indicator is calculated using the formula.
- Photoelectrocalorimetry. With this method, the color result of the reaction with blood plasma is obtained. The intensity of the color determines the amount of electrolytes.
The determination method used in a particular laboratory depends on its equipment.
Electrolyte standards for adults and children
The norm of components in the blood is different in children and adults. So, their main electrolytes have the following indicators.
IronPhosphorusPotassium
| Children | 7-18 mmol/l | 1.19-2.78 mmol/l | 3.5-5.5 mmol/l |
| Adults | 17.9-22.5 mmol/l | 1.87-1.45 mmol/l | 3.4-5.5 mmol/l |
Deviation of the indicator from these norms is a violation and harms the body. When deciphering the analysis result, an individual approach is important, as this allows us to take into account the characteristics of a particular patient.
Elevated electrolyte levels: causes
The reasons for the increase in the level of electrolytes in the blood can only be accurately determined after a complete examination of the patient. Most often, the disorder is caused by tumor processes, poor diet, overwork and infectious diseases such as tuberculosis. Problems in the functioning of the hormonal system and severe intoxication also provoke changes in the blood picture.
Reduced electrolyte levels: causes
Heart disease can cause electrolyte imbalance
A drop in electrolyte levels is also quite common. This disorder can be provoked by physical overload, nervous tension, alcohol and coffee abuse, as well as poor nutrition. The disorder can also develop against the background of liver pathologies, intestinal dysfunction, heart disease, and kidney disease.
How to normalize electrolyte levels
Normalizing the blood picture necessarily requires a balanced diet, the correct amount of physical activity and determining the cause of the deviation from the norm. If treatment is necessary, it is carried out depending on the diagnosis under the strict supervision of a doctor.
Source: https://gidanaliz.ru/analiz/elektrolity-krovi.html
Methods for determining the amount of electrolytes
The norm of electrolytes is isolated separately for each and determined in several ways:
- atomic-spectral method, in which the analyzed samples are transferred from a liquid state to “atomic vapor” by heating them (temperature of several thousand degrees);
- gravimetric method, in which serum samples are examined by a reaction that results in the formation of a precipitate, then it is weighed;
- a method of photoelectrocolorimetry, which makes it possible to achieve the desired color reaction of a solution with a blood sample; a conclusion is made based on its color saturation.
Water balance is determined using a special device - an electrolyte analyzer. It shows the content of potassium, sodium, calcium ions, blood plasma pH. The analyzer is equipped with electrodes that, due to their different installations, make it possible to determine only the level of potassium and sodium or all particles.
Blood test for electrolytes - what is it? Blood test norms
Electrolytes in the blood, their normal ratios, are the main condition for muscle contraction of the myocardium, and, consequently, life itself.
When many readers familiar with technology and chemistry hear the word “electrolyte,” the first thing that comes to mind is the liquids contained in the battery, batteries, and other power sources.
In fact, electrolytes are found in all living things without exception, since each cell requires the movement of individual particles, leading to metabolism.
More advanced compounds, such as proteins and enzymes, are immersed in the cytoplasm, the very basis of which, as well as the intercellular fluid, is an electrolyte.
Electrolytes include the simplest ions known to us from inorganic chemistry and possessing an electrical charge. These ions are capable of creating an electric current, which is based on the entire functioning of the nervous system and sensory organs. They promote the absorption of nutrients, stimulate metabolism and remove metabolic products from the body through the kidneys.
Only thanks to blood electrolytes do the cells contain as much water as needed, and there is a stable acid-base balance in the body.
Basic electrolytes transport water molecules from the blood and intercellular fluid into cells and back, they maintain osmotic balance and equality of concentrations in certain proportions, they stimulate or inhibit enzyme systems depending on need. What are the main electrolytes in our body, and what role do they play?
Basic electrolytes and their functions
The main protozoan, positively charged cations are sodium, potassium, which are monovalent, divalent magnesium and calcium cations, and the negatively charged chlorine anion. Their functions are:
- sodium is the main component of extracellular fluid, it retains the required volume of water in the body, the isolation of nerve impulses depends on it, and it is also the main substance that ensures the constancy of the balance of other electrolytes;
- Potassium is the most important component of the intracellular environment. In every living cell there is always more potassium than sodium, which is more abundant outside. It is potassium ions that stimulate any cellular action and impulses. Potassium ions provide electrical signals that are transmitted by nerves. It is potassium ions that trigger each beat of our heart, using a mechanism called spontaneous diastolic depolarization of the cells of the atriosinus node (pacemaker);
- chlorine is a negatively charged monovalent anion, and its main role is to form hydrochloric acid, which is produced in the stomach by parietal cells and takes an active part in digestion, being the main component of gastric juice;
- Magnesium is also necessary for the functioning of the muscular system, for the transmission of nerve impulses, for energy metabolism and for the metabolism of neurons. Magnesium is a calcium antagonist, and prevents the precipitation of its salts into an insoluble precipitate, thus preventing the formation of calcifications in the body;
- Calcium is mainly deposited in the form of phosphates in bone tissue. It is also necessary for proper muscle function, for the absorption of iron, takes part in the work of many enzymes and regulates blood clotting.
Thus, electrolytes work in pairs, being mutual antagonists of each other: sodium and potassium, calcium and magnesium.
Blood test for electrolytes - what is it?
The norms of blood electrolytes are quite narrow in their range, since it is from the concentration of inorganic compounds that the secondary parameters of the main environment of the body are produced, against the background of which all other biochemical processes unfold. The most important of the electrolytes listed are sodium and potassium.
If their mutual relationship is disrupted, then the fluid in the body is either retained or leaves. In case of dehydration, the concentration of these ions increases significantly, resulting in disturbances in the functioning of the heart, kidneys, musculoskeletal system and striated muscles, arrhythmia and convulsions appear.
In order to understand that this disorder is caused by a change in the concentration of electrolytes in the blood plasma, these biochemical studies of the concentrations of Na, K, Cl, Mg, Ca are used. What are the indications for studying blood plasma electrolytes? These are the following conditions in which there are electrolyte imbalances:
- profuse diarrhea and vomiting, exposure to hot climates, which leads to severe sweating, serious burns affecting a large area;
- in case of disturbances of acid-base balance - metabolic acidosis and alkalosis;
- when severe swelling occurs;
- in the presence of nagging muscle pain, cramps;
- in the event of extrasystole, atrial fibrillation, and other rhythm disturbances;
- if the patient, especially the elderly, is at risk of overdose of diuretics;
- to monitor the condition of patients with chronic kidney and heart diseases, especially chronic renal and congestive heart failure;
- with lethargy, drowsiness, stupor, stupor, various disorders of consciousness;
- for disorders of mineral metabolism in bones, osteoporosis;
- if the patient has endocrine pathology (hyperparathyroidism, diabetes insipidus).
There are many other indications that the doctor determines in each specific case. What is the normal level of electrolytes in the blood of a healthy adult?
Norms of blood electrolytes and reasons for deviations from reference values
The table of blood plasma ion balance indicators in the absence of pathology should have the following range of values:
| Element | millimoles per liter, mmol/l |
| potassium | 3,5-5,1 |
| sodium | 136 — 145 |
| chlorine | 98-107 |
| magnesium | 0,66-1,07 |
| calcium | 2,1 – 2,55 |
The indicated electrolyte standards do not include some age-related features that may be required when analyzing children. What are the most common causes of deviations from the norm? Here they are:
Sodium
An upward change in sodium values appears with endocrine pathology, with the consumption of large amounts of salt in food, with long-term use of drugs such as corticosteroid hormones, androgens and estrogens, and in women with oral contraceptives.
A lack of sodium in the human body occurs when there is a lack of salt in food, with profuse diarrhea, sweating and vomiting; the same loss of water and sodium through the skin occurs during fever. Sodium is lost with large doses of diuretics, in diseases such as diabetes and chronic adrenal insufficiency, as well as in the case of severe liver and kidney diseases.
Potassium
Hyperkalemia, or an increase in the level of potassium in the plasma, primarily occurs due to various destructions of cellular structures. For viral hepatitis and destruction of liver tissue, for cytolysis and anemia, for burns, for various types of shocks, for acute renal failure, as well as for effective treatment with chemotherapy when tumors disintegrate.
Hypokalemia, or a lack of potassium ions in the blood, occurs with the development of metabolic alkalosis, or excessive alkalization, with diabetes insipidus, with frequent deep breathing.
Chlorine
In the clinic, an excess of chlorine is occasionally encountered, but a deficiency can be detected quite often. It occurs with profuse indomitable vomiting, when all the synthesized chlorine for gastric juice leaves the body, with water poisoning, overhydration and polydipsia, with indomitable thirst, when there is no dehydration.
Also, a lack of chlorine is caused by excessive intake of diuretics, when it is secreted into the urine, in severe traumatic brain injuries, and in metabolic acidosis. Chronic and long-term chlorine deficiency can be accompanied by pathology of skin appendages, baldness and tooth loss.
Our article “The norm of chlorine in the blood and the reasons for elevated levels” is devoted to the content of chlorine in the blood.
Calcium
Excess calcium in the blood is most often associated with hormonal disorders due to increased production of the parathyroid glands of the calcium-regulating hormone - parathyroid hormone, if metastatic bone damage or bone tumor causes its destruction. In this case, calcium is directly absorbed into the blood. Diffuse toxic goiter and thyrotoxicosis, tuberculous bone damage, as well as excess vitamin D lead to an increase in calcium levels.
Calcium deficiency is widespread in rickets in children, in menopausal osteoporosis in women associated with estrogen deficiency, in myxedema or hypothyroidism due to chronic pancreatitis, when fat-soluble compounds containing vitamin D2 are not absorbed.
You can read more about the calcium content in the blood in our articles: “Normal calcium in the blood”, “Lack of calcium in the body: symptoms and signs”, “Ionized calcium: role in diagnosis, normal in the blood, reasons for increase and decrease”.
Magnesium
Conditions with increased magnesium are the opposite of calcium deficiency, and vice versa. But the most common are dehydration and taking diuretics, excessive intake of magnesium and antacids (they contain a lot of magnesium).
Its content in the blood decreases with hyperfunction of the thyroid gland, fasting and strict vegetarian mono-diets, intestinal diseases, as well as with chronic alcoholism.
Read more in the articles “Magnesium norm in a blood test: indications for analysis, causes of magnesium deficiency” and “Lack of magnesium in the female body: symptoms, causes, treatment.”
We also invite you to take a short test of 12 questions: Do you have enough magnesium? Test for women.
This short review listed the most important electrolytes in our body.
Currently, not a single major operation can be performed without their determination; patients in the intensive care unit undergoing dialysis are regularly tested for the content of electrolytes in the blood. Sometimes, in general outpatient practice, there is also a need to conduct such tests.
Source: https://MyAnaliz.ru/blood/analiz-krovi-na-elektrolity/
Potassium
Potassium is a positively charged ion found primarily inside the cells of all organs and tissues.
Potassium ensures nerve signal transmission and muscle contraction. Normally, a constant level of this ion is maintained in the blood and cells, but if the acid-base balance is disturbed, potassium can accumulate or be consumed, leading to hyperkalemia (increased potassium concentration) or hypokalemia (low potassium concentration). An increase or decrease in potassium concentration leads to disruption of the heart, water and electrolyte balance, paralysis, muscle weakness, and impaired intestinal motility. Indications for blood tests
for potassium levels:
- Assessment of kidney function in the presence of diseases of this organ;
- Assessment of acid-base balance;
- Cardiovascular diseases;
- Arrhythmia;
- Arterial hypertension;
- Adrenal insufficiency;
- Monitoring the concentration of potassium in the blood while taking diuretics and cardiac glycosides;
- Carrying out hemodialysis;
- Detecting deficiency or excess of potassium in the body.
The normal level of potassium in the blood of adults of both sexes is 3.5 – 5.1 mmol/l. In children, normal blood potassium concentrations depend on age and are as follows:
- Newborns up to 1 month – 3.7 – 5.9 mmol/l;
- Children 1 month – 2 years – 4.1 – 5.3 mmol/l;
- Children 2 – 14 years old – 3.4 – 4.7 mmol/l;
- Teenagers over 14 years old - like adults.
An increase in potassium levels in the blood is typical for the following conditions:
- Reduced excretion of potassium from the body in case of impaired renal function (acute and chronic renal failure, anuria, oliguria);
- Pathologies in which massive cell damage occurs (hemolytic anemia, disseminated intravascular coagulation syndrome, burns, trauma, rhabdomyolysis, hypoxia, tumor decay, long-term high body temperature, fasting);
- Intravenous administration of large amounts of potassium in the form of solutions;
- Metabolic acidosis;
- Shock;
- Diabetic coma;
- Decompensated diabetes mellitus;
- Dehydration (for example, due to vomiting, diarrhea, increased sweating, etc.);
- Chronic adrenal insufficiency;
- Pseudohypoaldosteronism;
- Addison's disease;
- Thrombocytosis (increased level of platelets in the blood);
- Increased muscle activity (for example, cramps, muscle paralysis after physical activity);
- Limiting sodium intake after strenuous exercise;
- Taking potassium-sparing diuretics and angiotensin-converting enzyme inhibitors.
A decrease in potassium levels in the blood is typical for the following conditions:
- Insufficient intake of potassium into the body (for example, during fasting, malabsorption, intravenous administration of large volumes of liquids with low potassium content);
- Loss of potassium through vomiting, diarrhea, through an intestinal fistula, wound, burn surfaces and intestinal villous adenoma;
- Cystic fibrosis;
- Taking non-potassium-sparing diuretics;
- Kidney failure;
- Renal acidosis;
- Fanconi syndrome;
- Primary and secondary hyperaldosteronism (excessive production of hormones by the adrenal cortex);
- Cushing's syndrome;
- Butter's syndrome;
- Infectious mononucleosis;
- Excessive urination, for example, with diabetes;
- Diabetic ketosis;
- Familial periodic paralysis;
- Administration of cortisone, testosterone, glucose, insulin, adrenocorticotropic hormone, vitamins B12 or folic acid;
- Low body temperature;
- Bulimia;
- Pancreatic islet cell tumor (PIToma);
- Magnesium deficiency.
Electrolytes in the blood: what they are, norm, definition, reasons for increase and decrease - About Blood
Electrolytes in the blood, their normal ratios, are the main condition for muscle contraction of the myocardium, and, consequently, life itself.
When many readers familiar with technology and chemistry hear the word “electrolyte,” the first thing that comes to mind is the liquids contained in the battery, batteries, and other power sources.
In fact, electrolytes are found in all living things without exception, since each cell requires the movement of individual particles, leading to metabolism.
More advanced compounds, such as proteins and enzymes, are immersed in the cytoplasm, the very basis of which, as well as the intercellular fluid, is an electrolyte.
Electrolytes include the simplest ions known to us from inorganic chemistry and possessing an electrical charge. These ions are capable of creating an electric current, which is based on the entire functioning of the nervous system and sensory organs. They promote the absorption of nutrients, stimulate metabolism and remove metabolic products from the body through the kidneys.
Only thanks to blood electrolytes do the cells contain as much water as needed, and there is a stable acid-base balance in the body.
Basic electrolytes transport water molecules from the blood and intercellular fluid into cells and back, they maintain osmotic balance and equality of concentrations in certain proportions, they stimulate or inhibit enzyme systems depending on need. What are the main electrolytes in our body, and what role do they play?
Electrolytes concept
In order to understand what it is, it is important to know that electrolytes are present in the blood in the form of differently charged particles:
Particles with a “-” sign are compounds of bicarbonates, phosphates, chlorides, organic acids. Positive particles - magnesium, calcium, sodium, potassium compounds.
In plasma, electrolytes account for no more than 1%, but in the body their role is significant.
The permeability of the cell membrane depends on the location of cations and anions and their quantitative composition. They also participate in the process of removing waste products from cells and promote the penetration of nutrients inside.