Composition and release form
Results can be seen seven days after using Rozart
The main active component in Rozart is rosuvastatin. Hyperomellose, lactose, and triacetin were used as additional substances.
Round tablets with a white or pink coating. On one side there is an engraving “ST1, 2, 3, 4”, the number indicates the dose of the active component. The drug is available in dosages of 5, 10, 20 and 40 milligrams of rosuvastatin.
Packed in cardboard boxes of 30 or 90 pieces. Each box must contain instructions for using the medicine.
Drug interactions
Transport protein inhibitors
Rosuvastatin is a substrate of several transport proteins, including the membrane transporter OATP1B1, involved in hepatic uptake, and the transport protein BCRP.
Concomitant use of rosuvastatin with drugs that inhibit these transport proteins may lead to an increase in the concentration of rosuvastatin in the blood plasma and increase the risk of developing myopathy.
The simultaneous use of rosuvastatin and cyclosporine does not affect the plasma concentration of cyclosporine, however, the effect of rosuvastatin is enhanced (its elimination slows down, AUC increases by 7 times, Cmax by 11 times). Concomitant use of cyclosporine and rosuvastatin is contraindicated.
Simultaneous administration of erythromycin and rosuvastatin leads to a decrease in the AUC of rosuvastatin by 20% and an increase in Cmax by 30%. This interaction may occur as a result of increased intestinal motility caused by erythromycin.
In patients receiving indirect anticoagulants (for example, warfarin), monitoring of the international normalized ratio (INR) is recommended, since starting therapy with rosuvastatin or increasing its dose may lead to an increase in the INR, and discontinuation of rosuvastatin or reducing its dose may lead to its decrease.
Gemfibrozil and other lipid-lowering drugs: simultaneous administration of gemfibrozil and rosuvastatin increases the Cmax and AUC of rosuvastatin by 2 times (see section Special instructions).
Based on specific interaction data, a pharmacokinetically significant interaction with fenofibrate is not expected, but a pharmacodynamic interaction is possible.
Gemfibrozil, fenofibrate, other fibrates and lipid-lowering doses of nicotinic acid (at least 1 g/day) increased the risk of myopathy when used concomitantly with other HMG-CoA reductase inhibitors, possibly due to the fact that they can cause myopathy and when used in as monotherapy.
When taking rosuvastatin simultaneously with one of the drugs in this group, patients are recommended to have an initial dose of rosuvastatin of 5 mg; a daily dose of rosuvastatin of 40 mg is contraindicated in this case.
The simultaneous use of rosuvastatin and antacids containing aluminum and magnesium hydroxide leads to a decrease in the plasma concentration of rosuvastatin by approximately 50%.
This effect is less pronounced if antacids are used 2 hours after taking rosuvastatin. The clinical significance of this interaction has not been studied.
The simultaneous use of rosuvastatin and oral contraceptives increases the AUC of ethinyl estradiol and AUC of norgestrel by 26% and 34%, respectively, which should be taken into account when selecting the dose of oral contraceptives.
There are no pharmacokinetic data on the simultaneous use of rosuvastatin and hormone replacement therapy; therefore, a similar effect cannot be excluded when they are used together.
The results of in vivo and in vitro studies showed that rosuvastatin is neither an inhibitor nor an inducer of cytochrome P450 isoenzymes. Rosuvastatin is a non-core substrate for these isoenzymes.
There were no clinically significant interactions with drugs such as fluconazole (inhibitor of CYP2C9 and CYP3A4 isoenzymes), ketoconazole (inhibitor of CYP2A6 and CYP3A4 isoenzymes), associated with metabolism with the cytochrome P450 system.
Co-administration of 10 mg rosuvastatin and 10 mg ezetimibe in patients with hypercholesterolemia resulted in a 1.2-fold increase in rosuvastatin AUC. However, a pharmacodynamic interaction between rosuvastatin and ezetimibe in relation to the occurrence of adverse events cannot be excluded.
Although the exact mechanism of interaction is unknown, use of HIV protease inhibitors with rosuvastatin may result in a marked increase in rosuvastatin exposure.
A pharmacokinetic study of co-administration of 20 mg rosuvastatin with a combination drug containing two HIV protease inhibitors (400 mg lopinavir/100 mg ritonavir) in healthy volunteers resulted in approximately two-fold and five-fold increases in rosuvastatin AUC0-24 and Cmax, respectively.
MORE ABOUT: Clotrimazole ointment: instructions for thrush in women
There is no clinically significant interaction between rosuvastatin and digoxin.
Table 1 lists various types of interactions, including interactions requiring dose adjustment of rosuvastatin. The dose of rosuvastatin should be adjusted if concomitant use with other drugs that increase the systemic concentration of rosuvastatin is necessary.
If the expected increase in AUC is approximately 2-fold or greater, then the initial dose of rosuvastatin should be 5 mg once daily. The daily dose of rosuvastatin should be adjusted so that its systemic concentration, taking into account its increase, does not exceed that when taking a dose of rosuvastatin 40 mg in monotherapy.
For example, when taking gemfibrozil, the dose of rosuvastatin should not exceed 20 mg (1.9-fold increase in AUC) and 10 mg when taking the atazanavir/ritonavir combination (3.1-fold increase in AUC).
Table 1. Effect of concomitant use of drugs on rosuvastatin exposure (AUC, in order of decreasing significance)
Dosing regimen of the interacting drug Dosing regimen of rosuvastatin Change in AUC value of rosuvastatin
Cyclosporine 75-200 mg twice a day, 6 months 10 mg once a day, 10 days Increase 7.1 times
Atazanavir 300 mg/ritonavir 100 mg once a day, 8 days 10 mg, single dose Increase 3.1 times
Lopinavir 400 mg/ritonavir 100 mg twice a day, 17 days 20 mg once a day, 7 days Increase 2.1 times
Gemfibrozil 600 mg twice a day, 7 days 80 mg, single dose Increase 1.9 times
Eltrombopag 75 mg once a day, 10 days 10 mg, single dose Increase 1.6 times
Darunavir 600 mg/ritonavir 100 mg twice a day, 7 days 10 mg once a day, 7 days Increase 1.5 times
Tipranavir 500 mg/ritonavir 200 mg twice a day, 11 days 10 mg, single dose Increase 1.4 times
Dronedarone 400 mg twice a day Not applicable Increase 1.4 times
Itraconazole 200 mg once a day, 5 days 10 mg, single dose Increase 1.4 times
Ezetimibe 10 mg once a day, 14 days 10 mg once a day, 14 days Increase 1.2 times
Fosamprenavir 700 mg/ritonavir 100 mg twice a day, 8 days 10 mg, single dose No changes
Aleglitazar 0.3 mg, 7 days 40 mg, 7 days No change
Silymarin 140 mg 3 times a day, 5 days 10 mg, single dose No changes
Fenofibrate 67 mg 3 times a day, 7 days 10 mg, 7 days No change
Rifampin 450 mg once a day, 7 days 20 mg, single dose No changes
Ketoconazole 200 mg twice a day, 7 days 80 mg, single dose No changes
Fluconazole 200 mg once a day, 11 days 80 mg, single dose No changes
Erythromycin 500 mg 4 times a day, 7 days 80 mg, single dose Reduction by 28%
Baikalin 50 mg 3 times a day, 14 days 20 mg, single dose Reduction by 47%
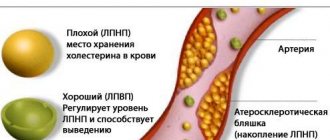
Effect of Rosart on high cholesterol
This remedy helps reduce the concentration of cholesterol in the body. Experts usually explain to patients the difference between good and bad cholesterol. The drug Rosart helps increase the content of good cholesterol. At the same time, the level of bad cholesterol decreases by almost 55% by the time the course of therapy is completed.
The strength of the product depends on its dosage. The patient will be able to notice the effectiveness of the drug within a week of regular use of the drug, and within a month the maximum result will be achieved.
Indications for use
There are several reasons to start using the drug, taking into account the required dosage. Namely:
- Prevention and treatment of atherosclerosis.
- If the diet is not effective in reducing excess cholesterol levels.
- Excessive levels of lipids in the blood.
- I or II degree of elevated cholesterol levels.
- Prevention of cardiovascular diseases in people at risk. We are talking about men over 60 and women over 50.
- Reducing the likelihood of recurrent stroke or heart attack.
- Elevated levels of C-reactive protein in the blood.
- Reduced levels of good cholesterol.
- Stably elevated blood pressure.
- Prevention of pathological changes in the functioning of the heart and blood vessels caused by addiction to tobacco and alcohol.
The hereditary factor completes the list of indications for prescribing Rozart tablets.
Indications for use
A specialist must prescribe the medicine
Indications for use of the product are the presence of pathologies such as:
- hypercholesterolemia – high level of cholesterol in the blood, which contributes to the development of gallstone disease, atherosclerosis, ischemia, obesity,
- hereditary form of hypercholesterolemia - elevated lipid levels are caused by abnormalities in chromosome 19. This pathology is inherited from both or only one parent,
- hypertriglyceridemia – ultra-high fat content in the human body,
- for preventive purposes to prevent the development of atherosclerosis and pathologies of the cardiovascular system.
Important! The specialist must prescribe the medicine, choose the dosage, and determine the duration of therapy. Remember that self-medication can cause undesirable consequences.
Methods of application
Description of the use of a medication for high cholesterol index with the main active ingredient rosuvastatin - Rozart:
- The beginning of drug therapy with the drug Rozart begins with a hypocholesterol diet, which accompanies the entire course of statin therapy;
- The attending doctor will tell you how to take Roseart, and the dosage is individually selected by the doctor in accordance with the biochemistry indicators with the lipid spectrum (lipograms);
- The Rosart tablet should be drunk whole and not chewed, and washed down with a large volume of water. There is no need to tie the medication to meals, you just need to observe the exact time of daily intake. It is recommended to take Rozart in the evening before bed, and this is associated with bioprocesses in the human body, and the time of active synthesis of cholesterol by liver cells;
- The initial dose of Rozart is 5.0 or 10.0 milligrams, once a day;
- Only the treating doctor can increase the dosage or replace the drug with an analogue, but not earlier than after a month of treatment with Rosart. The dosage is increased only based on the results of biochemical diagnostics and if the minimum dose is ineffective;
- The maximum dosage per day is 40.0 milligrams, prescribed to patients whose risk of developing cardiac pathologies or pathologies of the blood flow system is very high, but only if the medication Rozart with a dosage of 20.0 milligrams has not brought results in reducing the index cholesterol (for hypercholesterolemia of genetic or non-family etiology). Treatment with a Rosart dosage of 40.0 milligrams is carried out only in a hospital setting under the constant supervision of a doctor;
- The maximum dosage is also prescribed to patients who have a severe form of systemic atherosclerosis;
- During therapy with a dosage of up to 10.0 milligrams, monitor the cholesterol index and transminase levels after 14 days of use;
- With a mild degree of development of pathologies of the renal organ, there is no need to adjust the dose, and the dose is not adjusted in old age - older than 70 years, but treatment should be started with 5.0 milligrams per day;
- At a maximum dosage of 40.0 milligrams per day, constantly monitor the creatine phosphokinase index;
- If the patient’s medical history is at risk of developing myopathy, then treatment should be carried out with a dosage of Rosart of 5.0 milligrams;
- Patients with liver cell pathologies on the Child-Pugh scale, up to 7.0 points, should undergo a thorough diagnosis before prescribing and do not prescribe more than 5.0 milligrams per day.
The higher the dosage of the active component in the tablet, the greater the negative impact on the body from taking it.
The Rosart dosage of 40.0 milligrams causes the most side effects, so you need to start therapy with minimal dosages.
How and how much to take?
The tablets are swallowed whole with a sufficient amount of water. You can take them at any time, regardless of meals. Typically, experts prescribe a dose of 5 or 10 milligrams per day. It can be changed depending on the individual characteristics of the patient’s body.
The doctor prescribes the dose of the drug depending on the cholesterol level, the risk of developing a heart attack or stroke, and individual intolerance to the components of the drug. You can change the dosage once a month. The maximum permissible dose per day is 40 milligrams. Patients over the age of 70 are usually prescribed a dose of 5 milligrams.
To achieve greater effectiveness of therapy, it is best to carry it out in combination with an anti-cholesterol diet.
The substance rosuvastatin is absorbed by liver tissue. Almost 90% of the drug is excreted from the body in feces, and the remaining 10% is excreted in urine.
Price
Rosart tablets belong to the category of medium-cost drugs. Their price in pharmacies is determined depending on the number of tablets in the package and dosage.
| Dosage | Amount of drug | Average cost in Russia | Average cost in Ukraine |
| 5 mg | 30 pcs. | 500 rub. | 92 UAH |
| 40 mg | 90 pcs. | 900 rub. | 320 UAH |
| 5 mg | 30 pcs. | 1200 rub. | 370 UAH |
| 40 mg | 90 pcs. | 2000 rub. | 560 UAH |
Pregnancy and breastfeeding
Rosart should not be used by pregnant women
The drug Rozart is prohibited from use during pregnancy and breastfeeding. Fats are necessary for the normal formation of the fetus. For this reason, a woman expecting a baby cannot reduce her cholesterol levels. In addition, the drug is able to penetrate the placental barrier and cause skeletal disorders in the unborn baby. If a woman taking the drug finds out that she is pregnant, therapy must be interrupted immediately. Rosukart can be prescribed to women who use reliable contraceptives.
Pharmacological properties
Pharmacokinetics
Rosuvastatin is a selective, competitive inhibitor of HMG-CoA
reductase is an enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A into mevalonate, a cholesterol precursor.
The maximum concentration of rosuvastatin in blood plasma is achieved approximately 5 hours after oral administration. Absolute bioavailability is approximately 20%.
Rosuvastatin is absorbed primarily by the liver, which is the main site of cholesterol synthesis and LDL-C clearance. The volume of distribution of rosuvastatin is approximately 134 L.
Approximately 90% of rosuvastatin is bound to plasma proteins, mainly albumin. Rosuvastatin undergoes limited metabolism (about 10%).
In vitro metabolism studies using human hepatocytes indicate that rosuvastatin is a non-core substrate for metabolism by cytochrome P450 enzymes.
CYP2C9 is the main isoenzyme involved in metabolism. Isoenzymes 2C19, 3A4 and 2D6 are involved in metabolism to a lesser extent. The main identified metabolites of rosuvastatin are N-desmethyl and lactone metabolites.
The N-desmethyl metabolite is approximately 50% less active than rosuvastatin; lactone metabolites are pharmacologically inactive. More than 90% of the pharmacological activity in inhibiting circulating HMG-CoA reductase is provided by rosuvastatin.
About 90% of the dose of rosuvastatin is excreted unchanged from the body through the intestines (including absorbed and non-absorbed active substance of rosuvastatin), the remainder is excreted in the urine.
About 5% is excreted unchanged in the urine. The plasma half-life is approximately 19 hours. The half-life does not increase with higher doses of the drug.
The geometric mean plasma clearance is approximately 50 L/h (coefficient of variation 21.7%). As with other HMG-CoA reductase inhibitors, the process of hepatic uptake of rosuvastatin involves a membrane cholesterol transporter, organic anion transport protein C (OATP-C), which plays a major role in the elimination of rosuvastatin by the liver.
Systemic exposure of rosuvastatin increases in proportion to the dose. There are no changes in pharmacokinetic parameters when taking the drug several times a day.
Pharmacodynamics
Rozart reduces elevated LDL cholesterol, total cholesterol, triglycerides (TG), and increases high-density lipoprotein cholesterol (HDL-C).
It also reduces apolipoprotein B (ApoB), non-HDL-C (total cholesterol minus HDL cholesterol), VLDL-C, VLDL-TG and increases apolipoprotein AI (ApoA-I).
The therapeutic effect is achieved within one week after the start of treatment and after 2 weeks reaches 90% of the maximum possible effect. Typically, the maximum possible therapeutic effect is achieved after 4 weeks and is maintained with further use of the drug.
Rosart is effective in the treatment of adult patients with hypercholesterolemia with or without symptoms of hypertriglyceridemia, regardless of their race, gender or age, as well as in the treatment of special categories of patients, such as patients with diabetes mellitus or patients with a hereditary form of familial hypercholesterolemia.
How to take for liver diseases?
Before prescribing cholesterol-lowering therapy, it is necessary to undergo a comprehensive examination. Thanks to the tests, the specialist will be able to assess the condition of the liver. The same procedure must be repeated 3 months after starting to take Rosucard.
It is necessary to compare the test results and if an increase in the activity of liver enzymes is detected, the dosage should be changed or the use of the drug should be completely discontinued.
Patients with acute liver pathologies should not use Rosukart because it puts a lot of stress on the liver. Especially when the pathology is in the acute phase of development, even the lowest dosage of the medicine cannot be used.
Side effects
In early studies with daily doses ≥8 mg, there were a few cases of rhabdomyolysis. More recent studies have not used doses greater than 40 mg per day.
When using Rozart, side effects are observed, as when using statins. Myalgia, joint pain, headache, gastrointestinal symptoms: diarrhea or constipation and nausea often occur.
In the aforementioned long-term LIVES study, the most common negative symptoms were increases in creatine phosphokinase (2.7%), transaminases (1.5 to 1.8%), and gamma-GT (1%). Increases in liver enzymes were slightly more common in these studies than among other statins.
Proteinuria was more common in these studies (8.1%) (among other statins: 7.5%). Two cases of rhabdomyolysis occurred in the LIVES study (one of which was equivocal); this long-term study used daily doses of 10 or 20 mg of Rosart.
In the UK, the Food and Drug Administration has looked at the issue of rhabdomyolysis in detail; According to their report, between 2003 and 2008, there were 51 cases of rhabdomyolysis (37 severe) associated with the use of Rosart.
Another harm that Rosart caused to patients was a change in the amount of blood elements. Research methods have established that it has an effect on erythropoiesis and leukopoiesis. Therefore, patients suffering from anemia should exercise increased caution when using the drug in the long term. Rosart in these cases should be taken only after careful consideration of the benefits/harms by the treating doctor.
How to take if you have impaired renal function?
For patients suffering from renal pathologies, the use of Rosucard is strictly prohibited. In case of minor disruptions in the functioning of this organ, the dosage must be carefully controlled. You can take no more than 40 milligrams per day.
The presence of mild or moderate renal dysfunction is not a reason for dose adjustment. Almost 10% of rosuvastatin is excreted from the body through the genitourinary system. For this reason, the kidneys may be damaged in case of overdose.
Contraindications and side effects
If you take Rosucard at a dose below 20 milligrams per day, almost no unwanted effects appear
The drug at a dosage of 5, 10 and 20 milligrams should not be taken by patients with the following characteristics:
- intolerance to the components of the drug,
- liver pathologies in the active phase,
- renal dysfunction,
- myopathy,
- women of reproductive age who do not use reliable contraception,
- during pregnancy and breastfeeding,
- under the age of 18,
- lactose intolerance or deficiency.
A dose of 40 milligrams is prohibited for patients with:
- development of myotoxicity due to the use of other inhibitors,
- hypothyroidism,
- renal failure,
- tendency towards alcoholism,
- additional intake of fibrates,
- representatives of the Mongoloid race.
The development of undesirable effects in some cases is quite pronounced. For this reason, when prescribing this medicine, the specialist must take into account all the characteristics of the patient. In addition, side effects may occur due to too high a dose. If you take Rosucard at a dose below 20 milligrams per day, almost no unwanted effects appear. With increasing dosage, the following may occur:
- from the nervous system: general malaise, insomnia, neuralgia, sometimes depression, memory impairment,
- from the digestive system: abdominal pain, constipation, diarrhea, nausea, vomiting. In some cases, hepatitis and jaundice develop,
- respiratory system: cough, pharyngitis. In rare cases, pneumonia and runny nose,
- from the cardiovascular system: increased blood pressure, rapid heartbeat,
- from the endocrine system: development of diabetes,
- from the musculoskeletal system: myopathy, muscle pain, destruction of muscle tissue,
- hives, itching, and rashes may appear on the skin.
Rosart
Use during pregnancy and breastfeeding
The drug Rozart is contraindicated during pregnancy and lactation.
The use of the drug Rozart in women of reproductive age is possible only if reliable methods of contraception are used and if the patient is informed about the possible risk of treatment to the fetus.
Since cholesterol and substances synthesized from cholesterol are important for fetal development, the potential risk of inhibiting HMG-CoA reductase outweighs the benefit of using the drug during pregnancy. If pregnancy is diagnosed during therapy with Rozart, the drug should be stopped immediately, and patients should be warned about the potential risk to the fetus.
There are no data regarding the excretion of rosuvastatin in breast milk, therefore, if it is necessary to use the drug during lactation, taking into account the possibility of adverse events in infants, the issue of stopping breastfeeding should be decided.
Use for liver dysfunction
In patients with liver failure on the Child-Pugh scale below 7 points, no dose adjustment is required. In patients with Child-Pugh scores of 8 and 9, a preliminary assessment of renal function should be performed. There is no experience with the use of rosuvastatin in patients with liver failure above 9 points on the Child-Pugh scale.
Use for renal impairment
For mild or moderate renal failure, no dose adjustment is required. An initial dose of 5 mg is recommended for patients with moderate renal failure (creatinine clearance less than 60 ml/min). For patients with moderate renal failure (creatinine clearance less than 30-60 ml/min), administration of the drug at a dose of 40 mg is contraindicated. Taking the drug Rozart is contraindicated in any doses in patients with severe renal failure (creatinine clearance less than 30 ml/min).
Use in children
Contraindicated in children under 18 years of age (efficacy and safety have not been established);
special instructions
Effect on kidney function
In patients receiving high doses of rosuvastatin (mainly 40 mg), tubular proteinuria was observed during urine dipstick analysis, which in most cases was transient. This proteinuria did not indicate acute kidney disease or progression of kidney disease. The incidence of post-marketing reports of serious renal adverse reactions was higher in patients receiving rosuvastatin 40 mg.
When using the drug Rozart at a dose of 40 mg, it is recommended to monitor kidney function indicators during treatment.
Effect on the musculoskeletal system
Myalgia, myopathy and, in rare cases, rhabdomyolysis have been reported with all doses of rosuvastatin, and particularly with doses greater than 20 mg. In very rare cases, rhabdomyolysis has been reported while taking HMG-CoA reductase inhibitors and ezetimibe. In this case, a pharmacodynamic interaction cannot be excluded, so caution should be exercised when taking them together. As with other HMG-CoA reductase inhibitors, the incidence of post-marketing reports of rhabdomyolysis associated with rosuvastatin was higher with the 40 mg dose.
Determination of CPK activity
Determination of CPK activity should not be carried out after intense physical activity or in the presence of other possible reasons for an increase in its activity, which may lead to incorrect interpretation of the results obtained. If the initial CPK activity is significantly increased, a repeat measurement should be taken after 5-7 days; therapy should not be started if a repeat test confirms the initial CPK activity (5 times higher than normal).
Before starting therapy
Caution should be exercised when prescribing Rozart, as well as when prescribing other HMG-CoA reductase inhibitors, to patients with existing risk factors for the development of myopathy/rhabdomyolysis. It is necessary to consider the balance between the expected benefit of therapy and the potential risk and conduct clinical monitoring throughout the course of treatment. If the initial activity of CPK is significantly increased (5 times higher than the ULN), then treatment with the drug should not be started.
During treatment
The patient should be informed to immediately report to the doctor the unexpected onset of muscle pain, muscle weakness or cramps, especially in combination with malaise and fever. In such patients, CPK activity should be determined. Therapy should be discontinued if CPK activity is significantly increased (more than 5 times the ULN) or if muscle symptoms are severe and cause daily discomfort (even if CPK activity is 5 times less than the ULN). If symptoms disappear and CPK activity returns to normal, re-prescribing Rozart or other HMG-CoA reductase inhibitors in lower doses should be considered with careful monitoring of the patient. Routine monitoring of CPK activity in the absence of symptoms is impractical.
Very rare cases of immune-mediated necrotizing myopathy have been reported with clinical manifestations in the form of persistent weakness of the proximal muscles and increased CPK activity in the blood serum during treatment or discontinuation of statins, incl. rosuvastatin.
There were no signs of increased effects on skeletal muscles when taking rosuvastatin and concomitant therapy. However, an increase in the number of cases of myositis and myopathy has been reported in patients taking other HMG-CoA reductase inhibitors in combination with fibric acid derivatives (including gemfibrozil), cyclosporine, nicotinic acid in lipid-lowering doses ≥1 g/day, azole antifungals, inhibitors proteases and macrolide antibiotics. Gemfibrozil increases the risk of developing myopathy when taken concomitantly with certain HMG-CoA reductase inhibitors, therefore the simultaneous use of gemfibrozil and rosuvastatin is not recommended. The ratio of expected benefits and potential risks should be carefully weighed when using the drug Rozart and fibrates or nicotinic acid in lipid-lowering doses ≥1 g/day together.
Taking the drug Rozart at a dose of 40 mg simultaneously with fibrates is contraindicated.
During treatment, especially during the period of dose adjustment of the drug Rosart, the lipid profile should be monitored every 2-4 weeks and, according to it, the dose of the drug should be changed if necessary.
Rosart should not be taken by patients with acute and severe symptoms of myopathy or with risk factors predisposing to the development of renal dysfunction and secondary rhabdomyolysis (for example, sepsis, arterial hypotension, major surgical interventions, trauma, severe metabolic disorders, severe endocrine disorders and severe disturbances in water and electrolyte balance, uncontrolled seizures).
Effect on liver function
Like other HMG-CoA reductase inhibitors, rosuvastatin should be used with caution in patients who abuse alcohol and/or have a history of liver disease. It is recommended to determine liver function indicators before starting therapy and 3 months after starting therapy. Taking Rozart should be stopped or the dose reduced if the level of hepatic transaminase activity in the blood serum is 3 times higher than the ULN.
In patients with hypercholesterolemia due to hypothyroidism or nephrotic syndrome, treatment of underlying diseases should be carried out before starting treatment with Rozart. During post-marketing surveillance of rosuvastatin, the incidence of reports of serious liver dysfunction (expressed primarily as increased liver transaminases) was higher when taking a dose of 40 mg.
Ethnic groups
During pharmacokinetic studies among patients of the Mongoloid race compared to Caucasians, an increase in the systemic concentration of rosuvastatin was noted.
HIV protease inhibitors
During co-administration of rosuvastatin and a combination of various HIV protease inhibitors with ritonavir, an increase in the systemic concentration of rosuvastatin is observed. The decrease in blood lipid concentrations should be carefully assessed, and the possible increase in rosuvastatin in the blood plasma should also be taken into account at the beginning of treatment and during the period of increasing the dose of the drug Rozart in patients with HIV taking HIV protease inhibitors. Concomitant use of HIV protease inhibitors is not recommended without dosage adjustment of rosuvastatin.
Interstitial lung disease
Isolated cases of interstitial lung disease have been reported with the use of certain HMG-CoA reductase inhibitors, especially over long periods of time. Manifestations of the disease may include shortness of breath, nonproductive cough and deterioration in general health (weakness, weight loss and fever). If interstitial lung disease is suspected, therapy with HMG-CoA reductase inhibitors should be discontinued.
Diabetes mellitus type 2
Some evidence suggests that HMG-CoA reductase inhibitors increase blood glucose concentrations and increase the likelihood of developing type 2 diabetes in some patients. However, this risk is outweighed by the ability of HMG-CoA reductase inhibitors to reduce the risk of vascular complications, so this fact is not a reason to interrupt treatment with rosuvastatin. It is necessary to establish clinical observation and conduct a biochemical blood test according to national standards in patients at risk of developing hyperglycemia (blood glucose concentration 5.6-6.9 mmol/l, BMI >30 kg/m2, triglyceridemia, arterial hypertension). One study of rosuvastatin reported an overall incidence of diabetes mellitus of 2.8% in the rosuvastatin group and 2.3% in the placebo group, primarily in patients with fasting glucose 5.6-6.9 mmol/L.
Lactose intolerance
The drug Rozart should not be taken by patients with lactose intolerance, lactase deficiency and glucose-galactose malabsorption, since it contains lactose monohydrate.
Impact on the ability to drive vehicles and machinery
No studies have been conducted to study the effect of rosuvastatin on the ability to drive vehicles and operate machinery. Patients should be careful when driving vehicles and engaging in potentially hazardous activities, as Dizziness may occur during therapy.
Overdose
In case of overdose, consult a doctor
Dosage must be carefully monitored. Confusion must not be allowed, as this can lead to an overdose. It can lead to the development of side effects.
In case of overdose, you must immediately contact the doctor who prescribed the course of treatment.
The use of hemodialysis for Rozart poisoning will not have the desired effect.
Analogs
Rozart has many analogues that have a similar composition and release form. These include:
- Crestor - manufacturer IPEr Pharmaceuticals, package price - 238 rubles;
- Mertenil - manufacturer Gedeon Richter-RUS, price from 435 rubles;
- Roxera - manufacturer KRKA, price from 405 rubles;
- Akorta - manufacturer Pharmstandard-Tomskkhimpharm OJSC, price from 447 rubles;
- Rozulip - manufacturer Egis, price from 588 rub.
Interaction with other drugs
The effect of the medication is enhanced if it is taken with cyclosporine, gemfibrozil, and protease inhibitors. In this case, the dose of Rozart should be reduced.
In combination with drugs such as erythromycin and antacids, the effect of the drug is reduced.
Taking Rosukart with immunosuppressants and antifungal drugs may cause renal failure.
When combined with anticoagulants, there is a risk of bleeding.
In combination with alcohol, serious disturbances in liver function may occur.
To prevent the occurrence of such undesirable consequences, the medicine should be prescribed only by a specialist. The patient must inform him about his diseases and the means taken to treat them.
Drug interactions
HIV protease inhibitors and ezetimibe may enhance their lipid-lowering effects. There is no pharmacologically significant interaction with Digoxin. During treatment, it is necessary to conduct a lipid profile analysis every 3 weeks when combined with other drugs.
Most fenofibrates and nicotinamide increase the risk of muscle pain and rhabdomyliosis. In this case, take Rozart in a dose of 10 mg according to the instructions.
Drug toxicity increases if Rozart is taken together with oral contraceptives. The influence of hormones on female reproductive function may also noticeably increase.
No significant pharmaceutical interactions are observed with:
- Ketoconazole;
- Fluconazole;
- Amphocentrin.
Most antibacterial drugs can lead to a decrease in the concentration in the blood of Roseart. Indirect coagulants, together with statins, provoke a disturbance in the blood picture.